Compositions and libraries comprising recombinant t-cell receptors and methods of using recombinant t-cell receptors
一种细胞受体、细胞的技术,应用在免疫疗法领域,能够解决限制适用性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
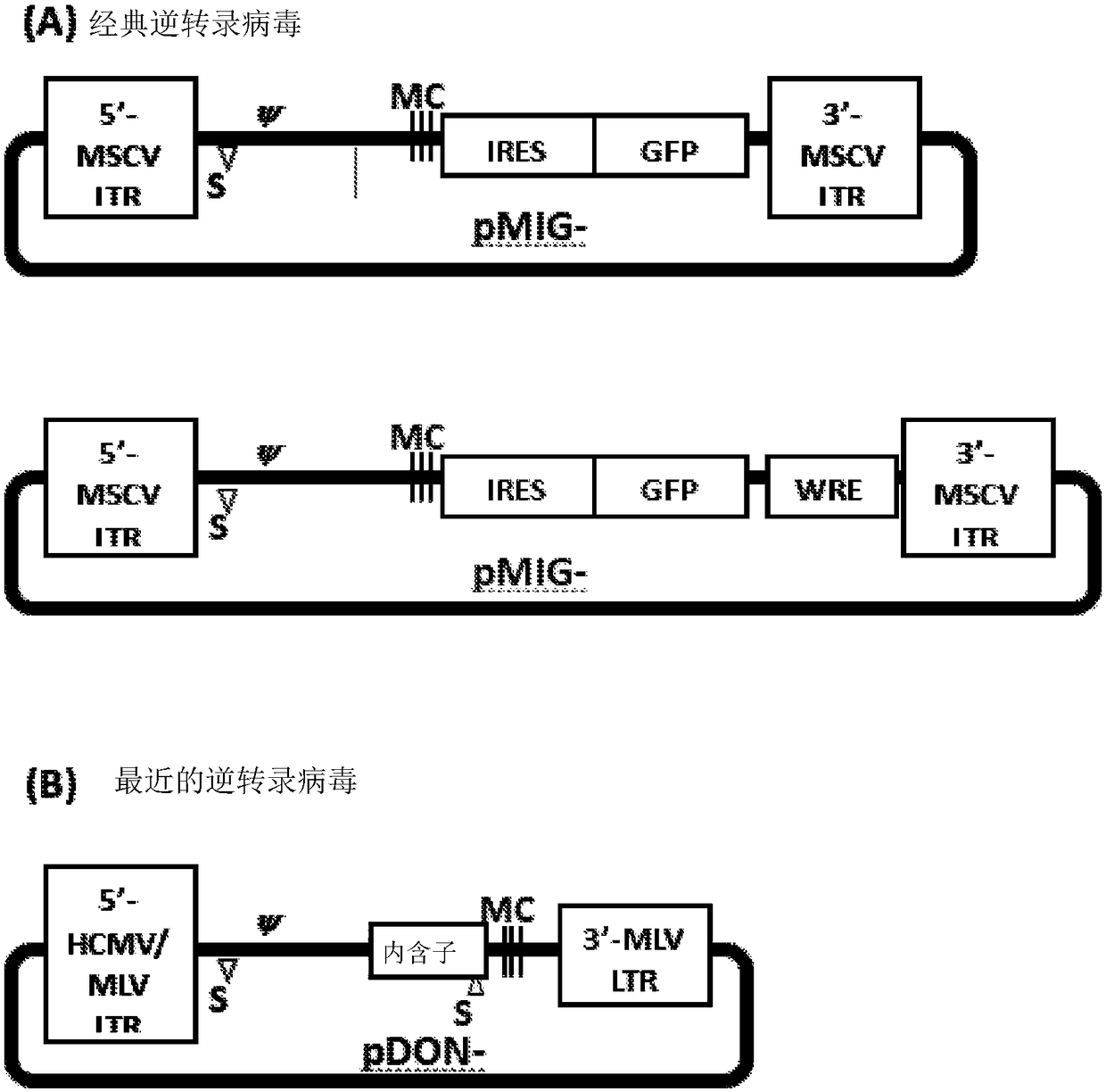
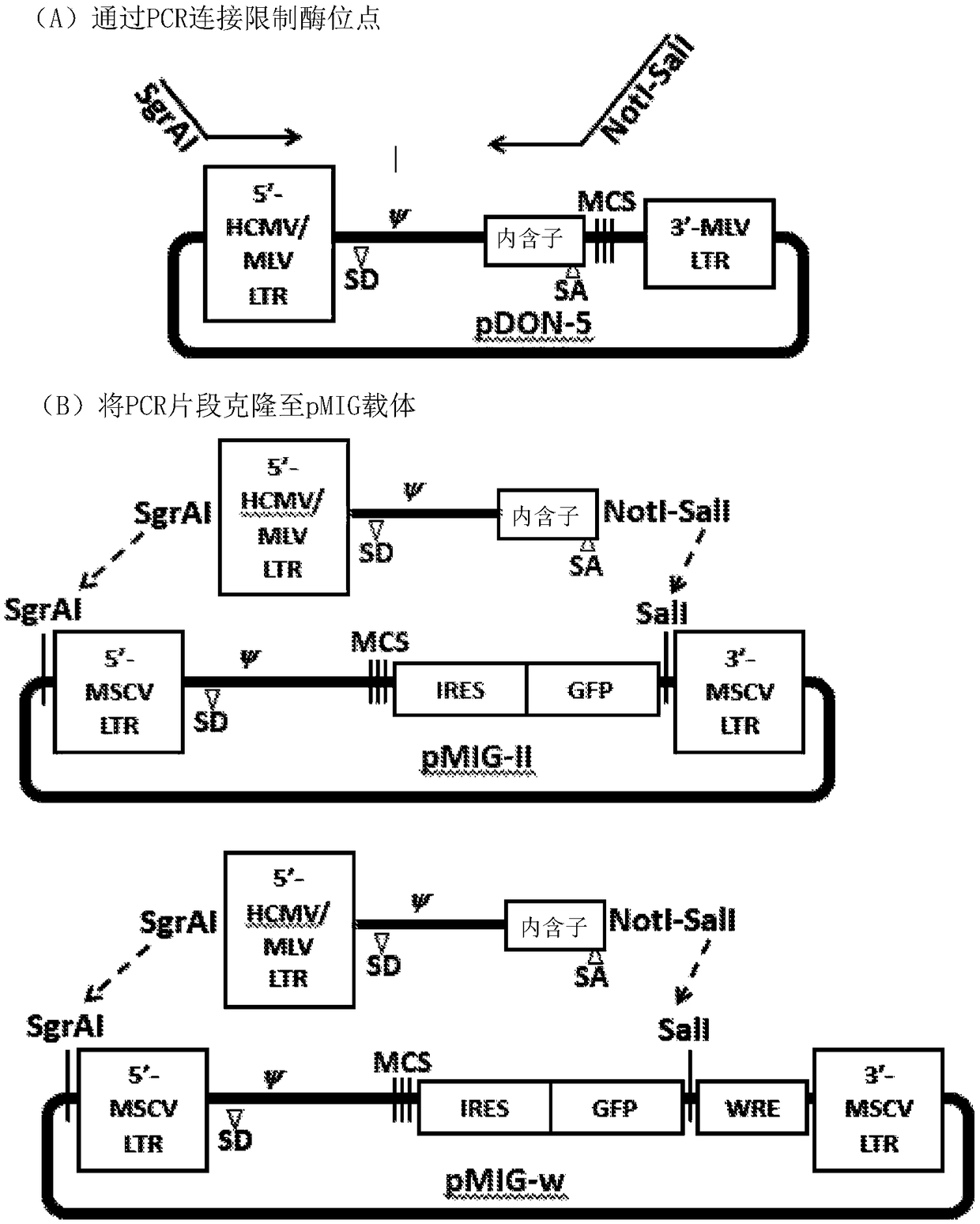
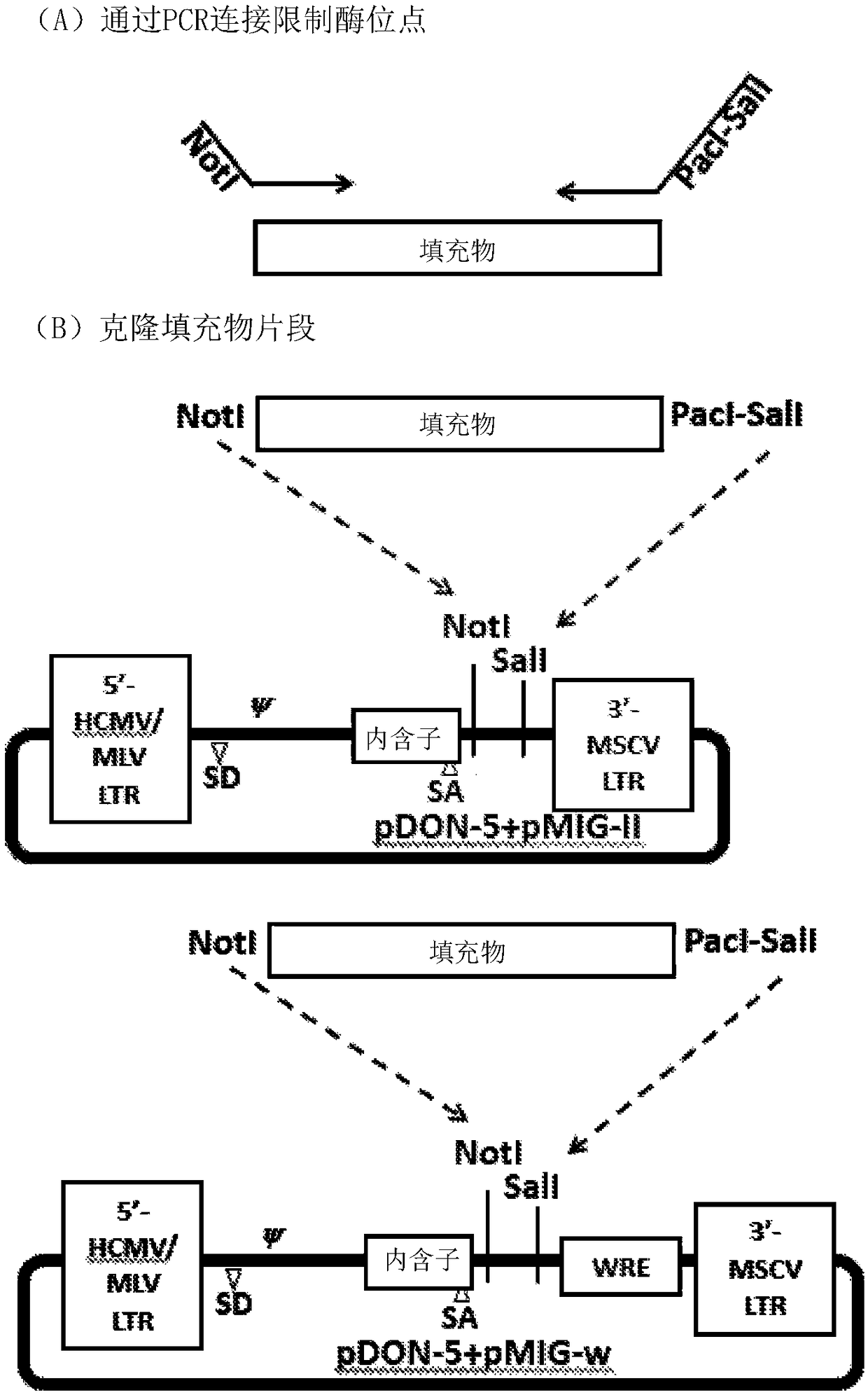
[0065] In specific and illustrative embodiments, the polynucleotide sequences encoding the TCRs of the present invention, as well as the amino acid sequences of the TCR alpha and TCR beta chains encoded by the polynucleotides, are as follows. Figures 1-4 show representative and non-limiting examples demonstrating TCR cloning and use.
[0066] HLA-A*02-Restricted NY-ESO-1 157-165 - specific T-cell clone "AL"
[0067] 1. Nucleotide sequence
[0068] TCR alpha chain
[0069] ATGATGAAATCCTTGAGAGTTTTACTAGTGATCCTGTGGCTTCAGTTGAGCTGGGTTTGGAGCCAACAGAAGGAGGTGGAGCAGAATTCTGGACCCCTCAGTGTTCCAGAGGGAGCCATTGCCTCTCTCAACTGCACTTACAGTGACCGAGGTTCCCAGTCCTTCTTCTGGTACAGACAATATTCTGGGAAAAGCCCTGAGTTGATAATGTCCATATACTCCAATGGTGACAAAGAAGATGGAAGGTTTACAGCACAGCTCAATAAAGCCAGCCAGTATGTTTCTCTGCTCATCAGAGACTCCCAGCCCAGTGATTCAGCCACCTACCTCTGTGCCGTGGGGGGACTTACCTCTAGCAACACAGGCAAACTAATCTTTGGGCAAGGGACAACTTTACAAGTAAAACCAGATATCCAGAACCCTGACCCTGCCGTGTACCAGCTGAGAGACTCTAAATCCAGTGACAAGTCTGTCTGCCTATTCACCGATTTTGATTCTCAAACAAATGTGTC...
Embodiment 2
[0207] This example provides a description of other TCR sequences that can be included in the libraries of the present disclosure along with any of the TCR sequences described in Example 1. These TCRs confer the ability of CD4+ T cells to directly recognize NY-ESO-1 / LAGE-1 positive cancer cells.
[0208] "JM" HLA-DPB1*0401 / 0402-restricted NY-ESO-1 157-170 - Specific tumor recognition of CD4 + T cell clones (a) cDNA nucleotide sequences of TCR alpha and beta chains
[0209] TCR alpha chain
[0210]ATGAAGTTGGTGACAAGCATTACTGTACTCCTATCTTTGGGTATTATGGGTGATGCTAAGACCACACAGCCAAATTCAATGGAGAGTAACGAAGAAGAGCCTGTTCACTTGCCTTGTAACCACTCCACAATCAGTGGAACTGATTACATACATTGGTATCGACAGCTTCCCTCCCAGGGTCCAGAGTACGTGATTCATGGTCTTACAAGCAATGTGAACAACAGAATGGCCTCTCTGGCAATCGCTGAAGACAGAAAGTCCAGTACCTTGATCCTGCACCGTGCTACCTTGAGAGATGCTGCTGTGTACTACTGCATCCCTAATAACAATGACATGCGCTTTGGAGCAGGGACCAGACTGACAGTAAAACCAAATATCCAGAACCCTGACCCTGCCGTGTACCAGCTGAGAGACTCTAAATCCAGTGACAAGTCTGTCTGCCTATTCACCGATTTTGATTCTCAAACAAATGTGTCACAAAGTAAGG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com