Methods of treating cancer with farnesyl transferase inhibitors
A technique of tipifarnib, dosing, applied in the field of cancer therapy, which can solve the problem of uneven response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0385] Clinical Study of Tipifarnib in Patients with PTCL
[0386]With the primary objective of evaluating the antitumor activity of tipifarnib, tipifarnib was administered in terms of objective response rate (ORR) in subjects with relapsed or refractory advanced peripheral T-cell lymphoma (PTCL) phase II clinical study. Determination of objective tumor response can be performed by International Working Group Evaluation Criteria (IWC) and / or measurable skin disease according to the Modified Severity Assessment Tool (mSWAT). Secondary objectives may include achieving effect of tipifarnib on progression-free survival (PFS) rate at 1 year, duration of response (DOR), overall survival (OS); and safety and tolerability of tipifarnib .
[0387] This phase II study investigated the antitumor activity of tipirfarnib with respect to ORR in PTCL subjects. Up to 18 eligible subjects with advanced PTCL were recruited. The total number of patients can be expanded to 30.
[0388] Sub...
Embodiment II
[0392] Evidence of Activity in Clinical Studies of Tipifarnib in Patients with PTCL
[0393] Evidence of clinical activity was studied in the patient cohort recruited in the study described in Example 1. Durable responses were observed in 4 of 8 PTCL patients (median 11 months).
[0394] The study was a Phase II study of 18 patients using a Simon two-stage design (11+7). 2 responses were required in the first 11 evaluable patients to proceed to Phase 2. If 5 responses were observed in Phase 1, enrollment was expanded to 30 patients.
[0395] In Phase 1, the 900 mg dose in every other week dosing, b.i.d., 7 days was changed to the 600 mg dose in every other week dosing, b.i.d., 7 days.
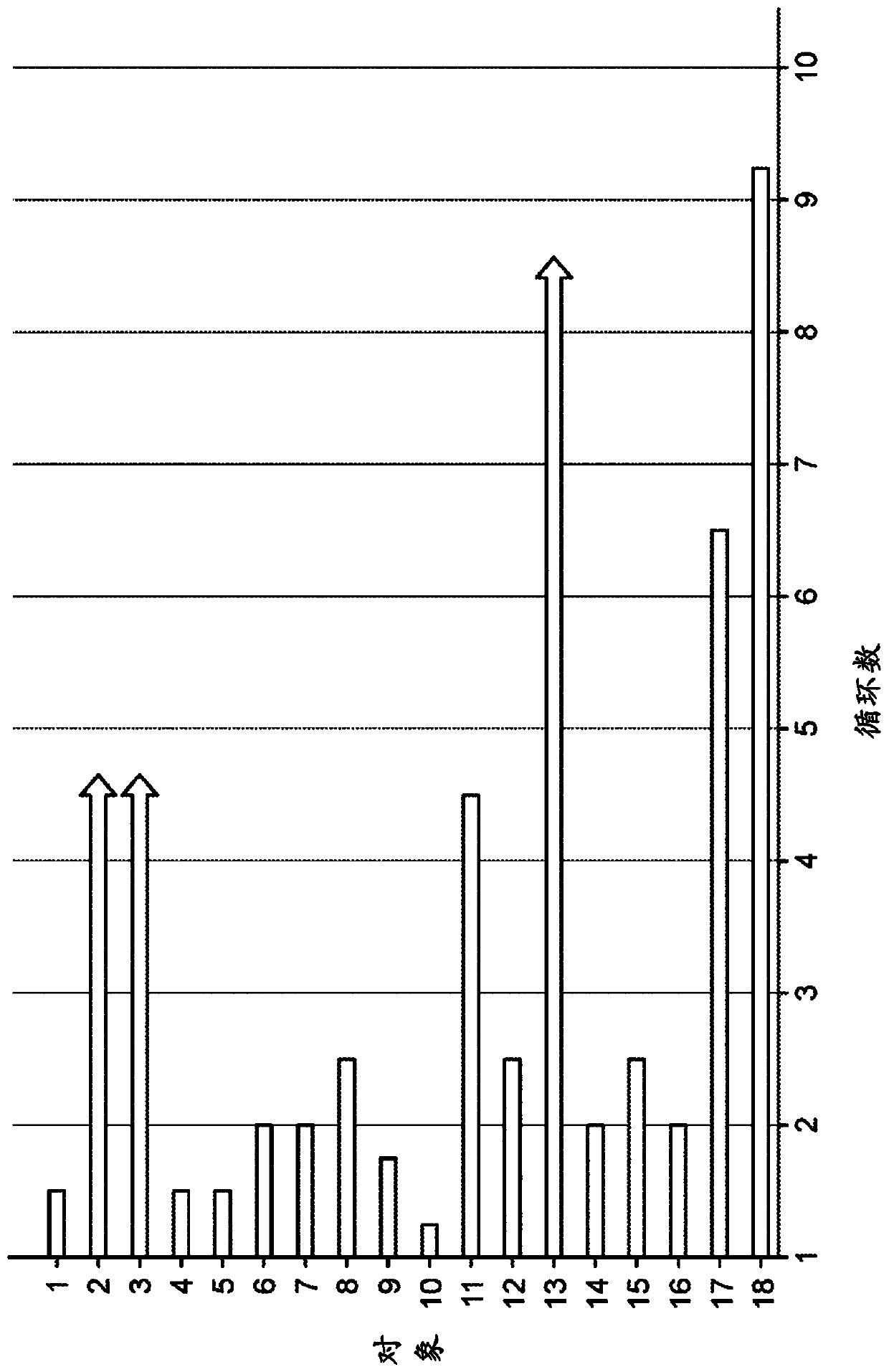
[0396] figure 1 The number of cycles received by each of the 18 patients dosed at the first time point during the study is shown. Table 1 lists each of the 18 patients, their PTCL type, and the study results. Three partial responses (PRs) were observed. Of the two AITL objects, both sh...
Embodiment III
[0409] Individualized FTI treatment decisions
[0410] The following procedures can be undertaken to determine a patient's suitability for FTI treatment, such as tipifarnib.
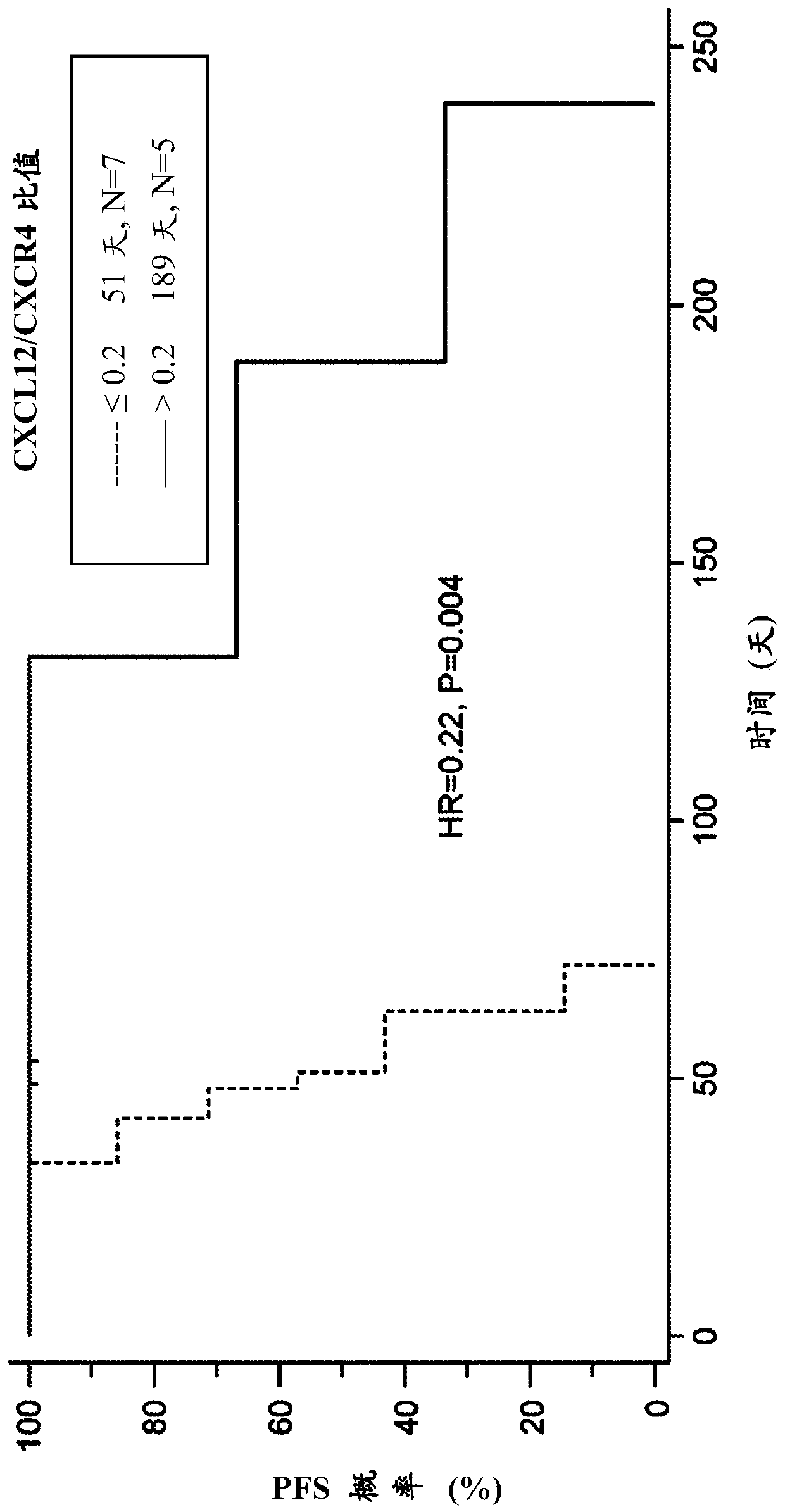
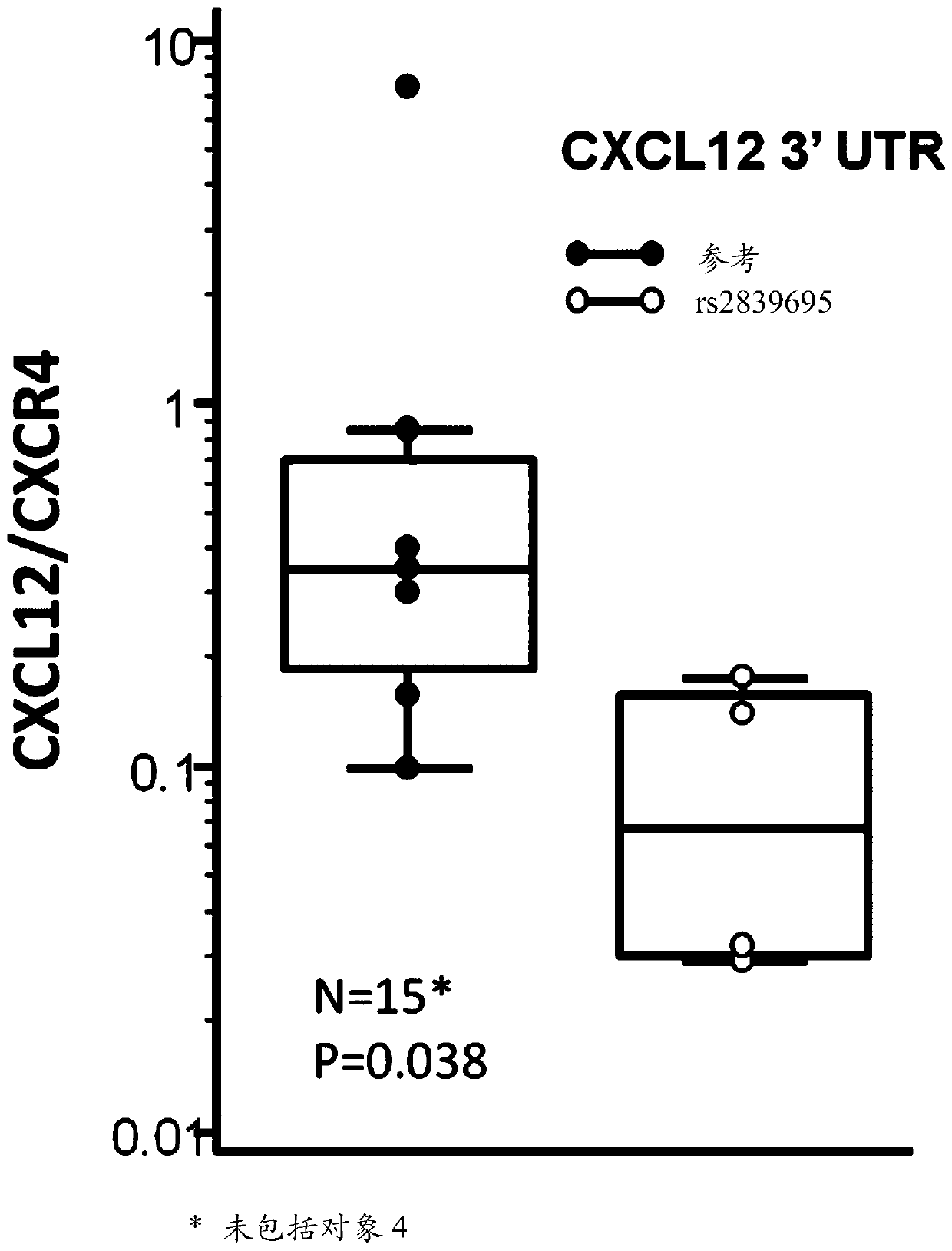
[0411] Antigen can be microwaved in 1-mmol / L concentration of EDTA, pH 8.0 using the standard indirect avidin-biotin-horseradish peroxidase method and diaminobenzidine chromogenic method, as is well known in the art. After repair, formalin-fixed paraffin-embedded tissue sections from patients were immunostained for CXCL12, CXCR4 and / or KIR3DL2 by human CXCL12, CXCR4 and / or KIR3DL2 monoclonal antibodies known in the art. For all cases studied, staining can be compared to a mouse IgG isotype control antibody diluted to the same protein concentration to confirm staining specificity.
[0412] Patients can also be tested for circulating CXCL12 in, for example, serum samples. Biopsy samples can also be tested for EBV biomarkers such as CXCL13 and PD-1.
[0413] T cells can be isolated from peripheral bloo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com