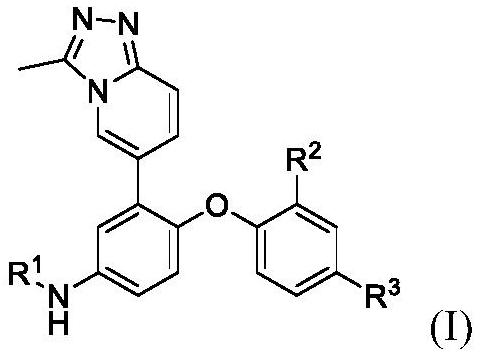
Triazole compound and its preparation method and application
A compound, the technology of triazole, applied in the field of medicine, can solve the problems of high neurotoxicity, poor water solubility, and restrictions on off-the-shelf drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1 Compound preparation
[0101] The synthetic route of compound of the present invention is as follows:
[0102]
[0103] The reagents used in the above preparation process are: (a) triethyl orthoacetate, 130 ° C; (b) sodium carbonate; tetrakis (triphenylphosphine) palladium; 2-fluoro-5-nitrophenylboronic acid, 1, 4-dioxane and water; 95°C; (c) cesium carbonate with R 2 , R 3 Substituent phenol, DMSO, 100°C; (d) 10% palladium carbon / H 2 ; methanol, room temperature; (e) pyridine, containing R 1 Sulfonyl chloride or acid chloride of the substituent, dichloromethane, 0°C to room temperature.
[0104] (1) Intermediate 2: Preparation of 6-bromo-3-methyl-[1,2,4]triazolo[4,3-a]pyridine
[0105] Dissolve 1.87g (10mmol) of 2-hydrazino-5-bromopyridine in 50mL of triethyl orthoacetate, raise the temperature to 130°C for 1 hour, pour the mixture into 100mL of ice water, and extract with ethyl acetate (60mL×3) Extract, combine the organic phases, dry the organic...
Embodiment 2
[0172] Example 2 : Detection of inhibitory activity of compounds on BRD4 protein
[0173] The inhibitory activity of compounds on BRD4 was detected by Homogeneous Time-Resolved Fluorescence (HTRF). The specific principles and experimental methods are as follows:
[0174] Experimental principle: HTRF combines the advantages of fluorescence resonance energy transfer FRET and time-resolved fluorescence TRF, and combines the homogeneous experimental method of FRET with the low background characteristics of TRF. It has the advantages of simple operation, high sensitivity, large throughput, The experimental data is stable and reliable.
[0175]Experimental procedure: Compounds were diluted with DMSO. Use the Diluent Buffer in the kit to dilute BRD4 (BD2, BD2) and Biotin-labeled histone H4 peptide, and prepare the reaction solution. Use DtectionBuffer in the kit to dilute Anti-GST-TB 2+ Cryptate and SA-XL-665, and configure the detection solution. Take a 384-well plate and arr...
Embodiment 3
[0183] Example 3 : EC of compound in J-Lat HIV-1 latent virus infected cell line 50
[0184] Experiment principle: EC 50 is the half-maximal effect concentration, which refers to the compound concentration corresponding to when the compound reaches 50% effect of activating HIV latent virus activity, and the unit is μM.
[0185] Experimental procedure: Take well-grown pseudovirus-infected J-Lat cells and spread them in 96-well transparent plates, and the amount of cells used is 2×10 per well. 5 Each, add different concentrations of the test compound (prepared according to the method of Example 1) respectively, the final concentrations are respectively 320, 160, 80, 40, 20, 10, 5, 0 μ M, JQ1 is the positive control group, and the untreated group is negative For the control, at least 3 replicate wells for each concentration, and each experiment was repeated 3 times. at 5% CO 2 After culturing in the incubator for 24 hours, collect the cells by centrifugation, discard the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com