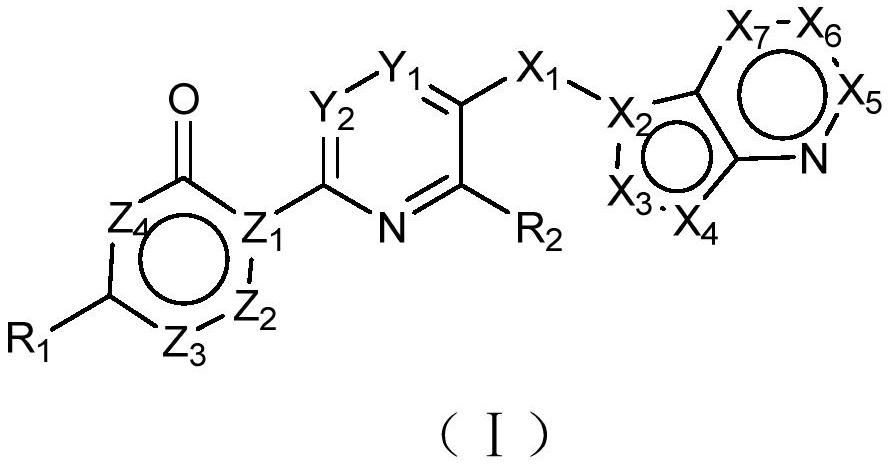
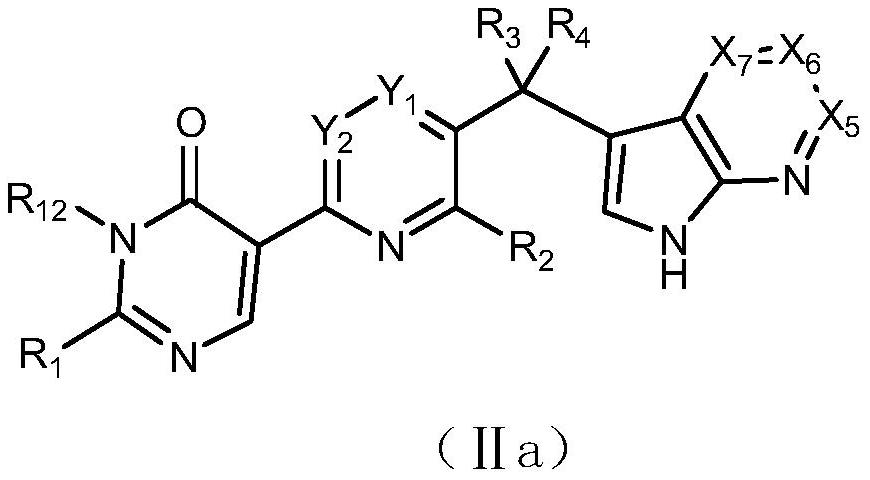
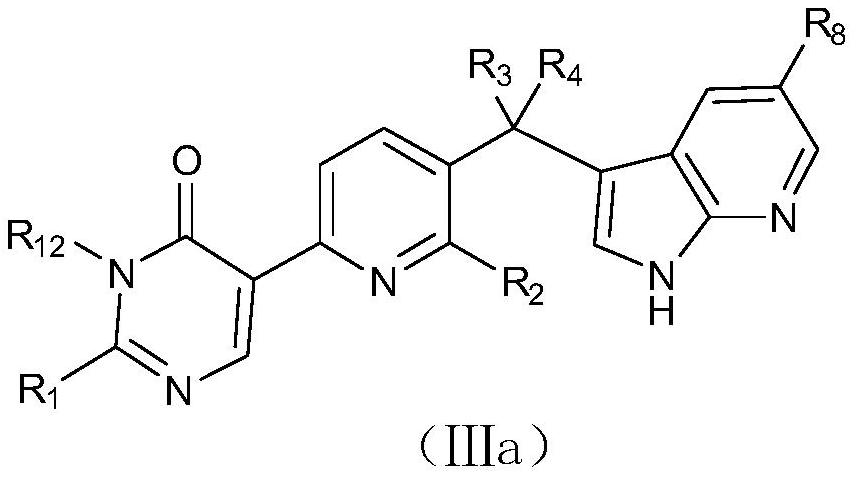
Azaheteroaryl derivative with csf1r inhibitory activity, preparation method and application thereof
A technology of heterocyclic group and alkyl group, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0192] 1. Preparation of intermediates
[0193] 1, Preparation of 5-bromo-N-isopropyl-4-methoxypyrimidin-2-amine (intermediate A1)
[0194]
[0195] 5-Bromo-2-chloro-4-methoxypyrimidine (2.4 g, 10.8 mmol), isopropylamine (6 mL, 72.4 mmol) and DIPEA (4 mL, 21.6 mmol) were added to the sealed tube, tetrahydrofuran (20 mL) was added, The reaction mixture was heated to 80°C for 5 hours. After cooling, the crude product was separated by column chromatography to obtain 5-bromo-N-isopropyl-4-methoxypyrimidin-2-amine (2.3 g, yield 88%).
[0196] 2. Preparation of (2-(isopropylamino)-4-methoxypyrimidin-5-yl)boronic acid (intermediate B1)
[0197]
[0198] 5-Bromo-N-isopropyl-4-methoxypyrimidin-2-amine (4.4g, 18mmol) was dissolved in dimethylformamide (30mL), and biboronic acid pinacol ester (13.7g, 53.9 mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (2.6g, 3.6mmol) and potassium acetate (10.6g, 107.8mmol), then evacuated to nitrogen at room temperature Thre...
Embodiment 1
[0301] Example 1, 5-(5-((1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-6-methylpyridin-2-yl)-2-(isopropylamino ) Preparation of pyrimidin-4(3H)-one
[0302]
[0303] 5-(5-((1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-6-methylpyridin-2-yl)-N-isopropyl-4-methoxy Pyrimidin-2-amine (30 mg, 0.08 mmol) was dissolved in acetic acid (3 mL), and 40% aqueous hydrobromic acid (0.085 mL, 0.6 mmol) was added. The reaction solution was stirred at 90°C for 6 hours. After the reaction solution was cooled, it was basified with saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, the organic phase was dried and concentrated, and separated by plate chromatography [eluent: dichloromethane to dichloromethane / methanol (10:1)] to obtain 5 -(5-((1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-6-methylpyridin-2-yl)-2-(isopropylamino)pyrimidine-4( 3H)-Kone (6.9 mg, 24% yield). MS m / z(ESI):375[M+H] + .
[0304] 1 H NMR (400MHz, DMSO-d 6 )δ11.44(s,1H),10.73(s,1H),8.74–8.57(m,1H),8.18(d,J=4.7Hz,1...
Embodiment 7
[0310] Example 7, 5-(5-((1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-6-methylpyridin-2-yl)-2-(isopropylamino )-3-methylpyrimidin-4(3H)-one preparation
[0311]
[0312] The first step: tert-butyl 3-((6-(2-(isopropylamino)-6-carbonyl-1,6-dihydropyrimidin-5-yl)-2-methylpyridin-3-yl )methyl)-1H-pyrrolo[2,3-b]pyridine-1-carboxylate
[0313]
[0314] 5-(5-((1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-6-methylpyridin-2-yl)-2-(isopropylamino)pyrimidine- 4(3H)-Kone (45 mg, 0.12 mmol) was dissolved in tetrahydrofuran (3 mL), di-tert-butyl dicarbonate (39 mg, 0.18 mmol) and N,N-diisopropylethylamine (46 mg, 0.36 mmol) were added , and the reaction solution was stirred at room temperature for 2 hours. The reaction solution was diluted with water, extracted with ethyl acetate, the organic phase was dried and concentrated, and separated by column chromatography [eluent: petroleum ether ~ petroleum ether / ethyl acetate (1:1)] to obtain tert-butyl 3-((6- (2-(isopropylamino)-6-carbonyl-1,6-dihyd...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap