Compounds for treating ocular diseases associated with excessive vascularization
An eye disease, compound technology, applied in sensory diseases, antibody medical components, fusion with soluble cell surface receptors, etc., can solve problems such as loss, retinal pigment epithelium shedding, irreversible damage to vision by photoreceptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0425] Example 1: Inhibition of choroidal neovascularization in a rat model of laser-induced choroidal neovascularization
[0426] Research design
[0427] Forty-eight (48) Brown Norway pigmented rats were divided into six (6) groups of eight (8) animals each. On day 0, choroidal neovascularization was induced in the right eye using a 532 nm argon laser photocoagulator (six (6) spots of 75 μm size, 150 mW, duration 0.1 sec). From day 0 (D0) just after induction with ChNV to day 21 (the last day of the study), the test items were administered by instillation 3 times a day or by oral twice a day. From day 0 to day 21 immediately after induction of neovascularization, the control item (vehicle) was instilled 3 times a day and the reference item (dexamethasone in olive oil) was administered daily by oral administration. Fundus neovascularization was assessed in the right eye on days 14 and 21 using Heidelberg retinal angiography (HRA). At the end of the in vivo phase, lesion ...
Embodiment 2
[0455] Example 2: Inhibition of VEGF and bFGF-induced neovascularization
[0456] Methods of assessing the area of neovascularization
[0457] The area of neovascularization can be measured using the following formula:
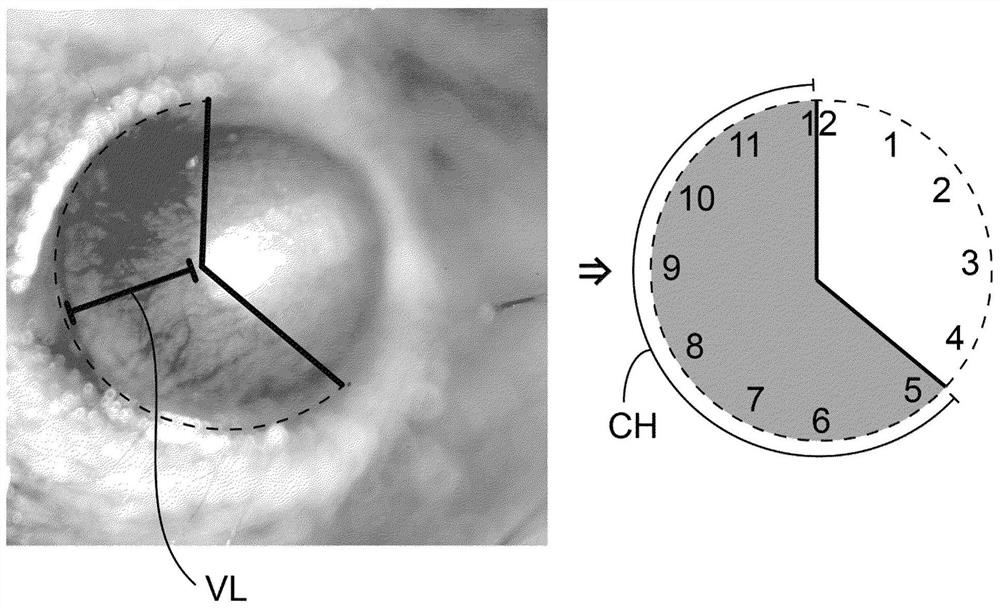
[0458] Area = 0.2·VL·CH·π
[0459] Among them such as figure 1 Vessel length (VL) and continuous circumferential area (expressed in clock hours = CH) were measured as defined in .
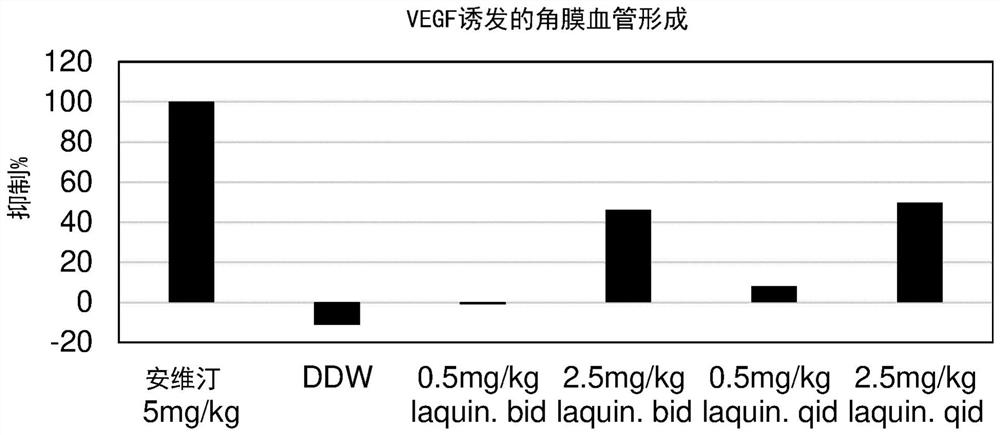
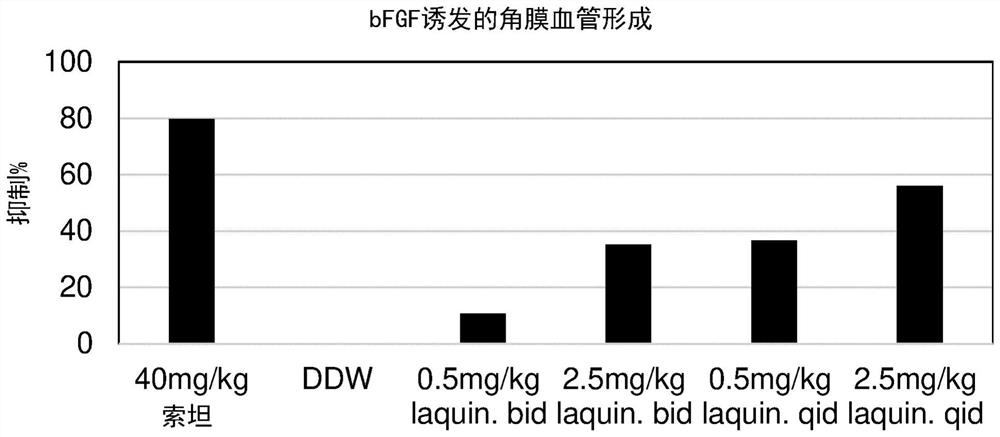
[0460] Treatment of VEGF-induced corneal angiogenesis with laquinimod
[0461] Hydron beads for inducing angiogenesis were prepared from a stimulating agent (VEGF) and a binding agent (sucralfate).
[0462] Anesthetized by intraperitoneal injection of 90 mg / kg pentobarbital, 53 CR female C57BL / 6 mice aged 6 to 8 weeks were prepared for surgery. Corneal vascularization was induced by placing the pellets in an incised corneal pocket in one eye. Monitor carefully for signs of eye irritation or infection.
[0463] Doses of laquinimod, vehicle (DDW = double distilled wat...
Embodiment 3
[0490] Example 3: Laquinimod and ABR-215174 have effects on LPS-activated microglia-induced tube formation in human retinal microvascular endothelial cells
[0491] method
[0492] The following experimental groups were included in the study:
[0493] Group 1: Control (vehicle, 0.1% DMSO)
[0494] Group 2: Sulforaphane (10 μM, positive control, anti-angiogenic effect)
[0495] Group 3: Aflibercept ("Aliya" 40μg / ml, positive control, partial anti-angiogenic effect)
[0496] Group 4: ABR-215174 (0.1 μM)
[0497] Group 5: ABR-215062 (10 μM)
[0498] Human retinal microvascular endothelial cells (HRMEC) were purchased from Neuromics (Cat. No. HEC09, Lot No. 2872) and grown at 37°C, 5% CO according to the manufacturer's instructions 2 Cultures were performed in Endo-Growth Medium (Cat. No. EKG001, Lot No. EKG0011902269) supplemented with Endothelial Growth Factor (Cat. No. EKG001, Lot No. EGK00125) under AlphaBiocoat-coated T25 flasks.
[0499] Primary human microglia from ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap