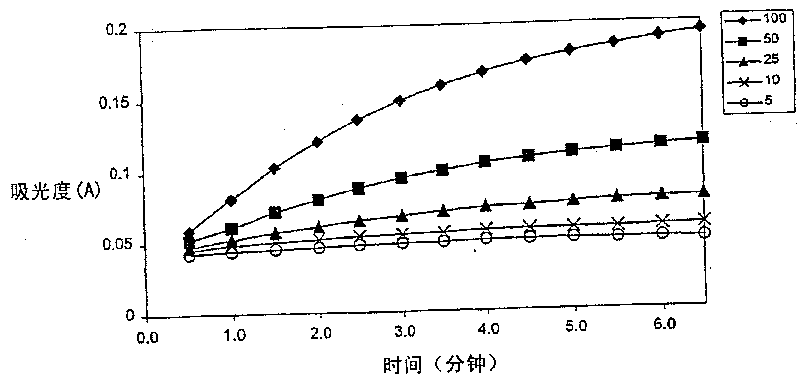
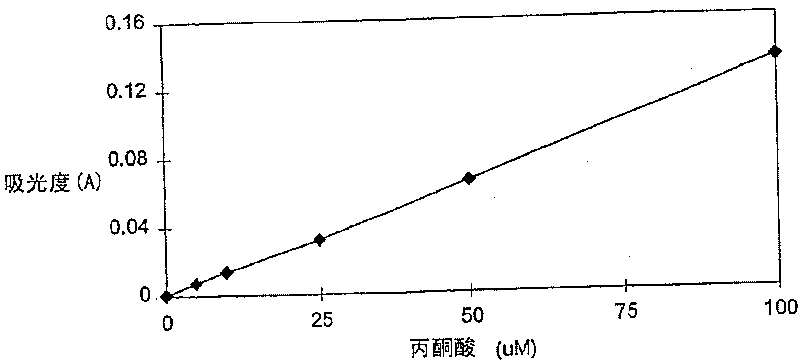
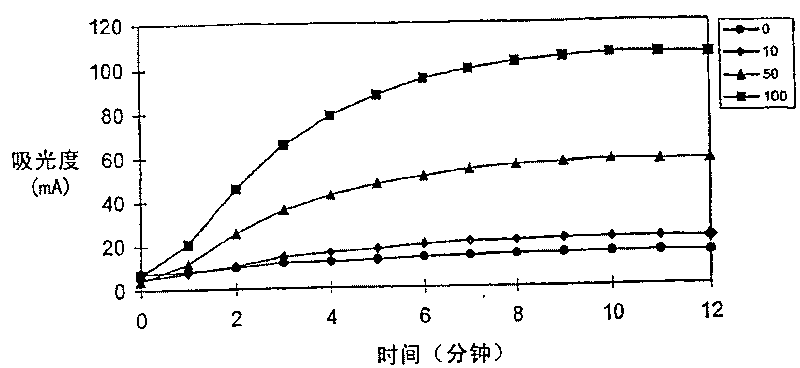
Enzymatic cycling assays for homocysteine and cystathionine
A technology of homocysteine and cystathionine, which is applied in the field of enzymatic cycling assay for homocysteine and cystathionine, can solve the problem of increasing the measurement sensitivity, reducing the steady-state concentration of the compound, no unreported Problems such as enzymatic cycling assays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Cloning and expression of cystathionine β-lyase gene from Escherichia coli
[0101] In this example, the E. coli CBL gene was cloned using standard PCR procedures. The isolated gene is inserted into an expression vector with an affinity marker sequence (6HIS), and the expressed protein is purified with an affinity column. Large amounts of CBL enzyme activity in host cells were transformed with expression vector constructs and purified from cell lysates.
[0102] Cloning of Escherichia coli cystathionine β-lyase (CBL, EC 4.4.1.8) gene
[0103] A standard PCR procedure used the Extended High-Fidelity PCR System (Roche Biochemicals) for amplification of the E. coli CBL gene, with ONE SHOT TOP10 competent E. coli cells (Invitrogen) as the source of genomic DNA. The PCR primer sequences used for CBL cloning were as follows: (CBL N-terminal primer: 5'-CGACGGATCCGATGGCGGACAAAAAGCTTG-3'; SEQ ID NO: 1) and (CBL C-terminal primer: 5'-CGCAGCAGCTGTTATACAATTCGCGCAAA-3'; SEQ ID N...
Embodiment 2
[0110] Cloning and Expression of Yeast Cystathionine β-Synthase (CBS) Gene in Yeast
[0111] In this example, the PCR method was used to clone the CBS gene from yeast. The PCR fragment containing the CBS gene was inserted into the expression vector with and without an affinity tag, and the addition of the tag facilitated the purification of the expressed gene product.
[0112] A laboratory strain of S. cerevisiae (5153-11-1 (his3met2)) was used as the source of the CBS gene. Cells were grown on YEPD at 32°C, centrifuged, washed with water and transferred into 200 μl yeast plasmid buffer (100 mM NaCl, 10 mM Tris (pH 8), 1 mM EDTA, 0.2% SDS). The cell suspension was mixed with an equal volume of 0.45 mm glass beads and vortexed at high speed for 3x30 seconds. Liquid was removed by pipetting and centrifuged in a microfuge for 2 minutes to remove cell debris. The supernatant was extracted with PCI (phenol:chloroform:isoamyl alcohol (25:24:1) and chloroform. Sodium chloride (5...
Embodiment 3
[0123] Cloning, expression and purification of yeast cystathionine β-synthase (CBS) gene in Escherichia coli
[0124] In this example, standard PCR and molecular biology techniques were used to clone the Saccharomyces cerevisiae (INVSc1) cystathionine β-synthase (CBS) gene as a fusion with an amino-terminal glutathione-S-transferase (GST). protein. Addition of this glutathione-S-transferase domain allows for a single-step affinity purification of the CBS protein from E. coli culture medium and also assists in the solubilization of the active form of the enzyme and / or facilitates proper refolding.
[0125] clone
[0126] A laboratory strain of Saccharomyces cerevisiae (INVSc1) (Invitrogen) was used as the CBS gene source. A single colony of INVSc1 yeast was boiled in 100 μl of deionized water for 5 minutes and rapidly vortexed for 2 minutes in the presence of an equal volume of 0.45 mm glass beads. 1 μl of this Cell lysates were then used as a source of DNA for PCR amplific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com