Protein having tpo activity
A protein and activity technology, applied in the field of new proteins, can solve the problems of small content, uncompleted separation, biochemical and biological identification and characterization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 20
[0189] The human liver cDNA library (hTPO-F1) constructed in Example 20 was divided into 3 small libraries (library numbers 1-3#). PCR was performed using the plasmid DNA prepared from each small pool as a template and using the synthesized primers. As a result, when the plasmid DNA prepared from the 3# mini-pool was used, a DNA fragment with the expected size was amplified. The 3# small pool was then divided into sub-pools, each containing 15,000 transformants, and screened by PCR as described above. As a result, amplification of DNA with the expected size was found in 6 of the 90 small pools. When these positive small pools were divided into subpools, each containing 1,000 clones, plasmid DNA was extracted in the same manner and PCR was performed, no DNA amplification was observed. This is considered to be due to the low recovery rate of plasmid DNA caused by the weaker growth of the clone of interest than other clones. Therefore, the original 3# pool was plated in 100 LB...
Embodiment 18
[0198] The results of Examples 18 and 22 show that human TPO protein can still exhibit its biological activity even after removing the 3 amino acids at its C-terminus. Therefore, deletion derivative experiments were performed for further analysis of the biologically active fractions. A series of expression plasmids were prepared by PCR using the DNA of the plasmid clone pHT1-231 obtained in Example 18 as a template and synthetic oligonucleotides as primers.
[0199] The resulting expression plasmid contains the DNA encoding the human TPO deletion derivative, which lacks the C-terminal region of the TPO protein, that is, the deletion derivative encodes amino acid sequences at positions 1-211, 1-191, 1-171 and 1-163. When plasmid DNA was prepared from individual clones and transfected into COS1 cells, TPO activity was detected in each culture supernatant (Example 27).
[0200] Design a series of derivatives with deletion of C-terminal amino acid residues to position 151 and oth...
Embodiment 1-1
[0286] Purification induced by administration of antiplatelet antibodies
[0287] Rat TPO in plasma of thrombocytopenic rats
[0288] Preparation of plasma from rats with thrombocytopenia induced by administration of antiplatelet antibody
[0289] TRP was prepared as a purified source from approximately 1,000 rats according to the method described in the aforementioned Reference Example A Rat Megakaryocyte Progenitor Cell (CFU-MK) Assay System.
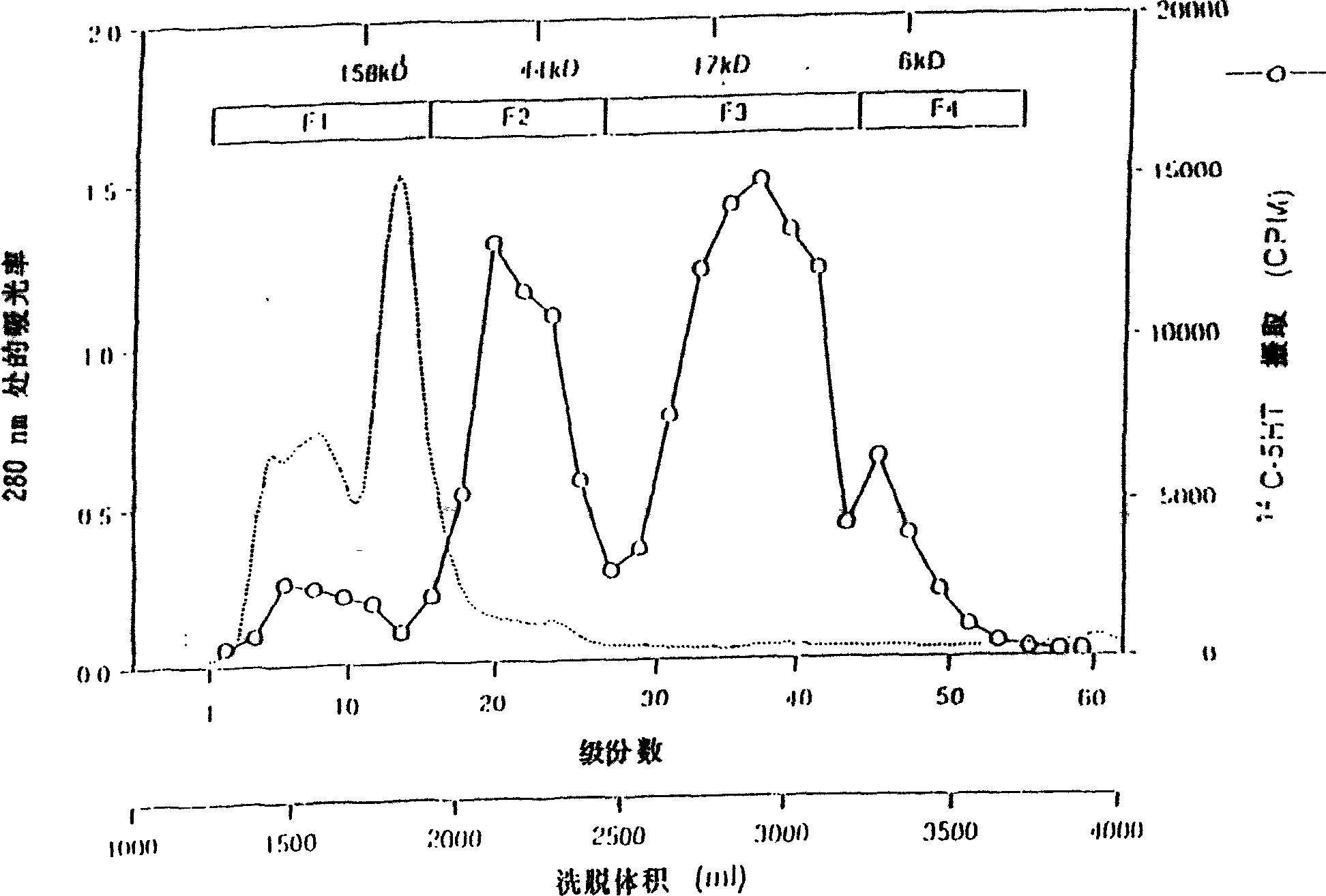
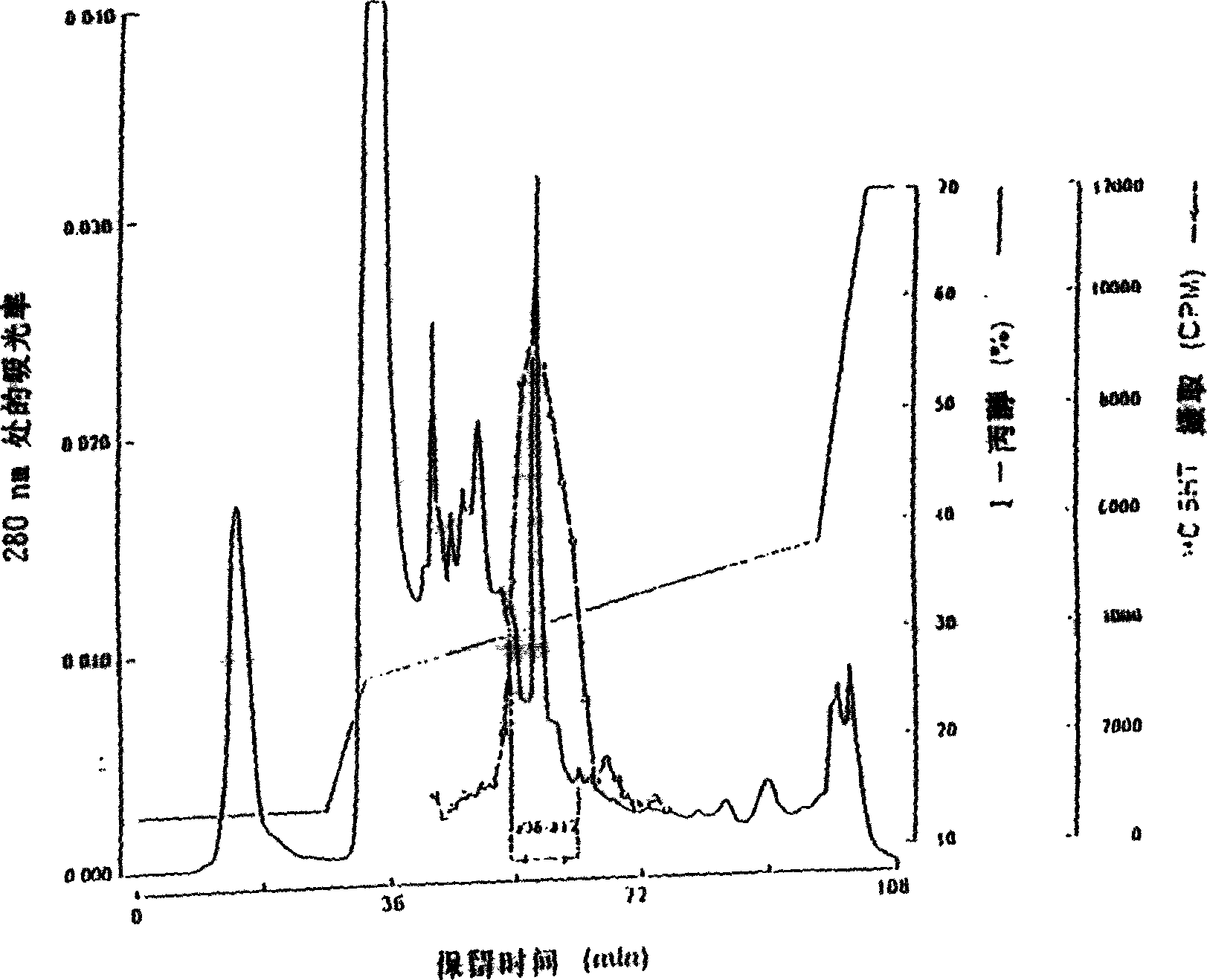
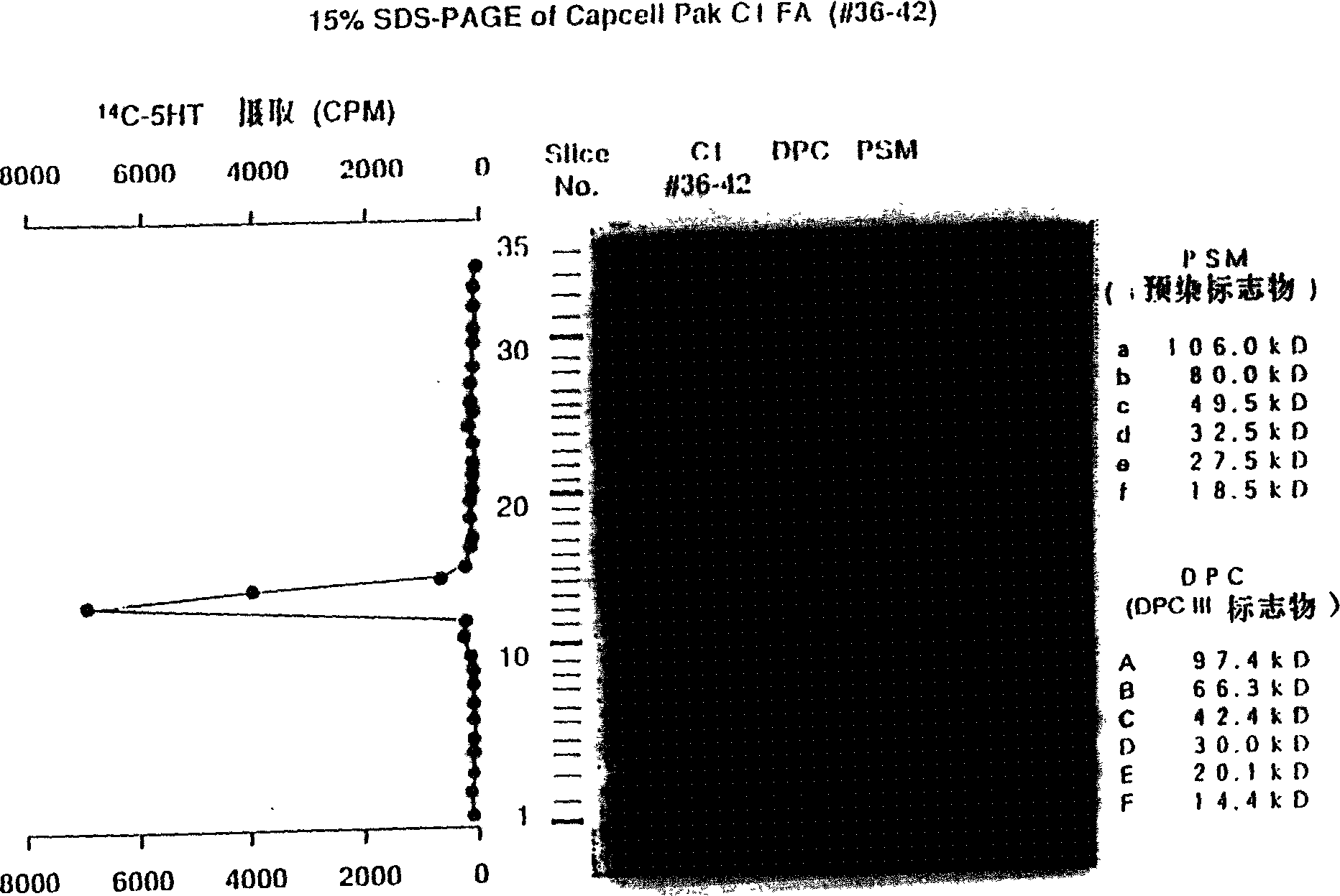
[0290] Purification of rat TPO from TRP
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com