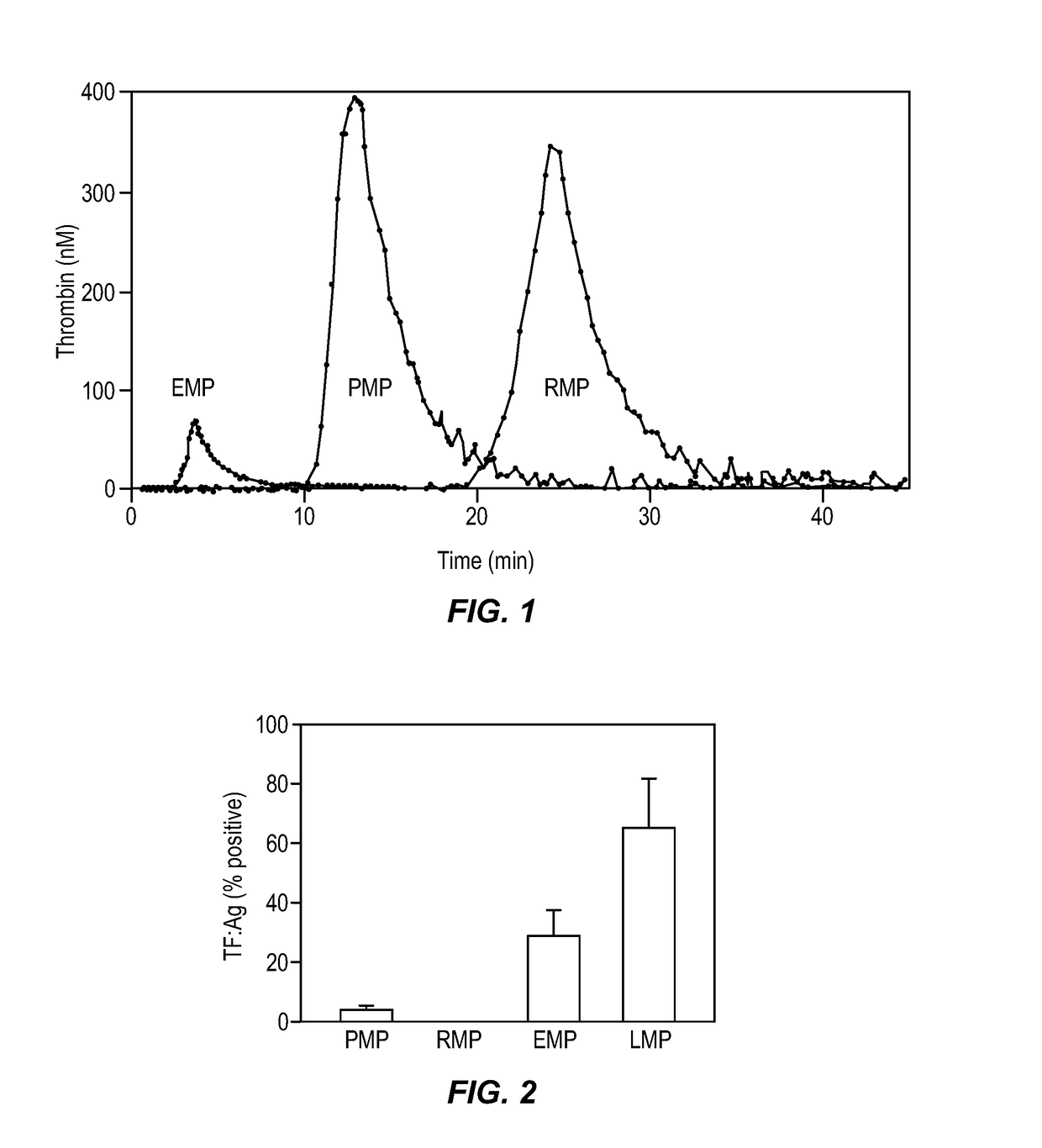
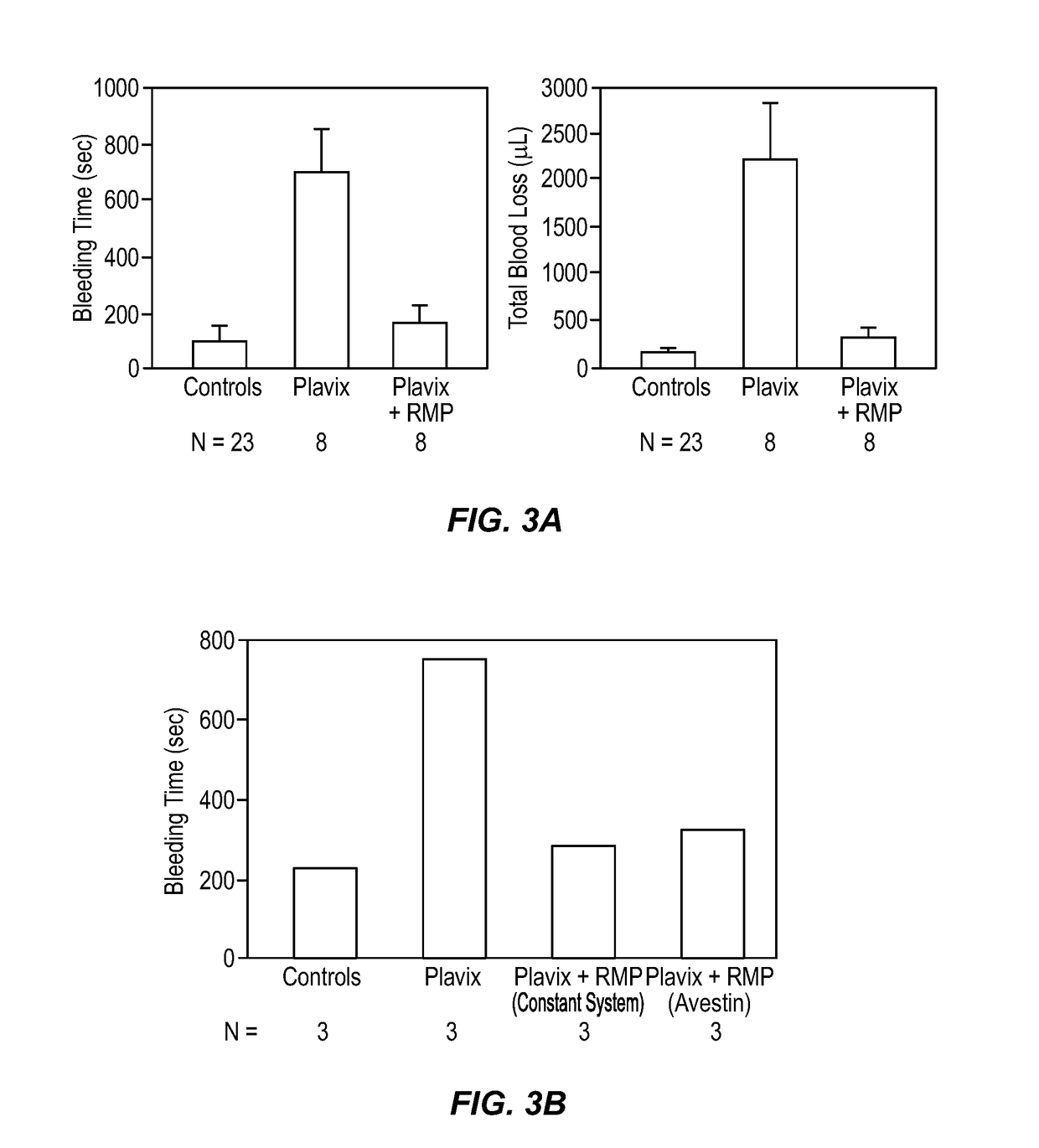
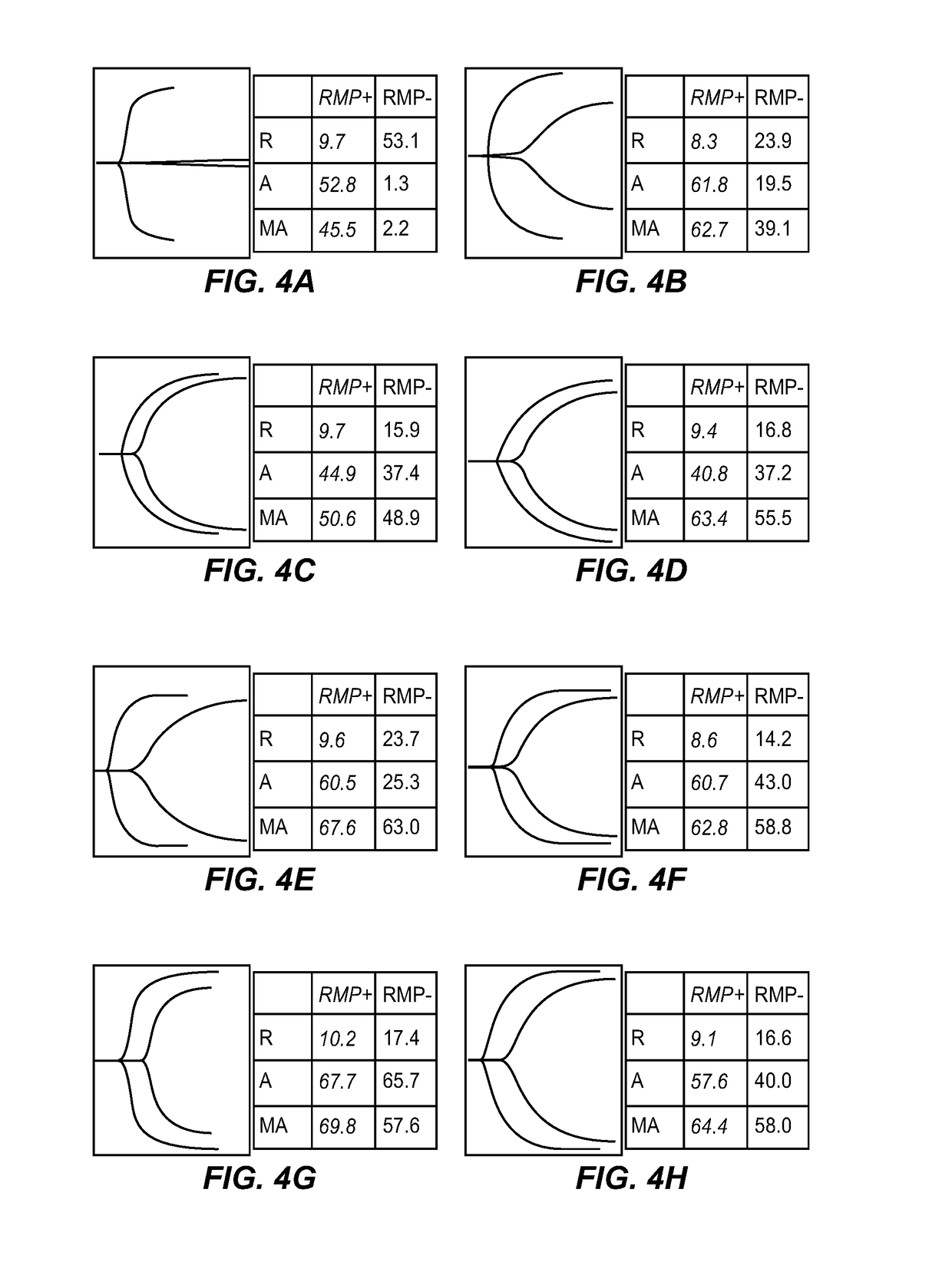
Expanded utility of red-cell derived microparticles (RMP) for treatment of bleeding
a technology of red cell derived microparticles, which is applied in the field of hematology, can solve the problems of merely replacing lost blood, unable to save the lives of many bleeding victims, and high cost of transfusion, so as to reduce the strain on limited supplies, not place additional burden on supplies, and prolong the shelf li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of RMP (Laboratory Scale)
[0044]For a single batch, 30 mL packed cells from blood bank are diluted with 60 mL of isotonic saline containing 1.5 mM EDTA pH 7.4. Cells are washed 3 times with the same EDTA / saline by centrifuging 15 min. each at 750×g at room temp. The final resuspension is brought to volume 60 mL, which is then drawn into the stainless-steel pressure cell of the French Press (Thermo-Electron Inc.) and then expelled at an internal pressure of 25,000 psi at rate 1.5 mL / min (achieved by adjustment of the needle valve). The resulting effluent was centrifuged at low speed (750×g) to remove the small number of unbroken cells, and the supernatant was then centrifuged at 18,000×g for 45 min. to sediment the RMP. One of ordinary skill in the art will appreciate that filtration and other separation techniques can be employed to remove the unbroken cells. The RMP were then washed at the same speed in isotonic saline (no EDTA) and refrigerated overnight prior to lyophil...
example 2
Potential for Scale-Up
[0045]This principle (shear induced by high-pressure extrusion) can be readily adapted to commercial-scale production. It was arrived at only after extensive experimentation with many other methods such as ionophore, sonic disruption, osmotic rupture, and others. The basic function of a French Press is to apply shear to fluid suspended cells. The cell suspension is placed in a pressure cell where pressure is applied by means of a hydraulic ram whereupon the pressurized suspension is forced (extruded) through the orifice of a needle valve into a region of atmospheric pressure. The great drop in pressure as the cells pass through the orifice applies great shear force to the biomembranes. The process is controlled by setting a target pressure on the hydraulic ram and controlling the needle valve to achieve a given flow rate. A drawback to the French Press is that the volume processed is limited by the size of the pressure cell (typically 100 mL) and the need to ma...
example 3
Lyophilization for Storage
[0046]An RMP batch (from Example 1) made the previous day was mixed with optimal amounts of albumin, glucose, sorbitol and / or trehalose, then pipetted in 1.0 mL aliquots into 2 mL lyophilizing vials, then placed in the tray of a Triad Freeze Dry System (Labconco, Inc.). The instrument operation is divided into programmable segments. Our experiments established the following optimal program settings. Samples are first subjected to a 4-hour “Pre-freeze” phase, with brief vacuum, during which temperature drops to −75° C. Segment 1. After switching to full vacuum (<0.07 mbars), temperature is ramped up (at 0.27° C. / min) to −30° C. and held for 2-hr. Segment 2. Temperature is slowly increased (at 0.04° C. / min) to −25° C., and held for 2-hr. Segment 3. Temperature is ramped to −15° C. at 0.04° C. / min, and held for 12-hr. Final Step. Vials are sealed in vacuum; rubber stoppers were pre-placed on the vials and pressed on to seal by pressure plate. After removing vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com