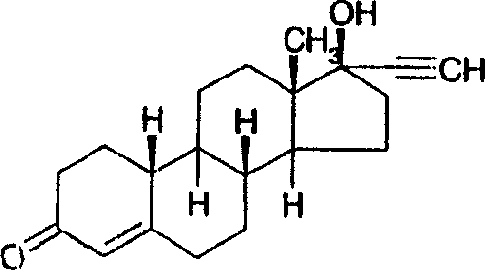
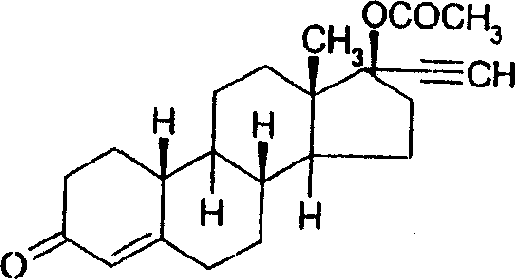
Norethindrone sustained release formulations and methods associated therewith
A technology of norethisterone and norethisterone acetate, which is applied in the directions of organic chemistry, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as increasing the risk of pregnancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The transdermal matrix system containing norethindrone and norethindrone acetate was prepared as follows. The solid content of the acrylic binder solution (Durotak 87-2074) is determined by placing a small amount in a pre-weighed aluminum dish and then placing it in a 75°C convection oven (A4718-Q, Blue M) overnight. After evaporating the solvent, the weight of the dry binder is obtained, and the solid content is calculated as the ratio of dry and wet weight.
[0062] Binder 87-2074 contains approximately 28-31% solids and has been used undiluted. Based on the pre-determined solid content, a known amount of binder is weighed into the glass bottle. For all preparations, first add an appropriate amount of norethindrone (NE) to the liquid binder in each bottle (1% w / w drug content is obtained after drying). Cap the bottle, seal with parafilm, and rotate until all NE is dissolved. Add an appropriate amount of norethindrone acetate (NEA) to the preparation bottle that does not r...
Embodiment 2
[0065] Using the adhesive matrix patch prepared according to the above process, the modified Franz diffusion cell was used to conduct in vitro skin flow studies. The epidermal membrane of a human cadaver is separated by heat. Cut the matrix patch of each formulation into 0.71 cm 2 Wafer. The release liner is removed, discarded, and the matrix sheet is laminated on the stratum corneum of the epidermal membrane. Then the skin-matrix assembly is sandwiched between the donor and recipient chambers of the diffusion cell and fixed so that the epidermis side faces the recipient chamber. Inject 0.02% w / v sodium azide (NaN 3 ) Solution. Then place the cuvette in a circulating water bath maintained at 32±1°C.
[0066] At 24, 48, 72, 96, 120, 144, and 168 hours, the entire contents of the recipient chamber were collected for drug quantitative analysis. Then reperfuse the receptor chamber with fresh receptor medium. After the HPLC analysis of the sample, the interval flow rate and cumulative ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com