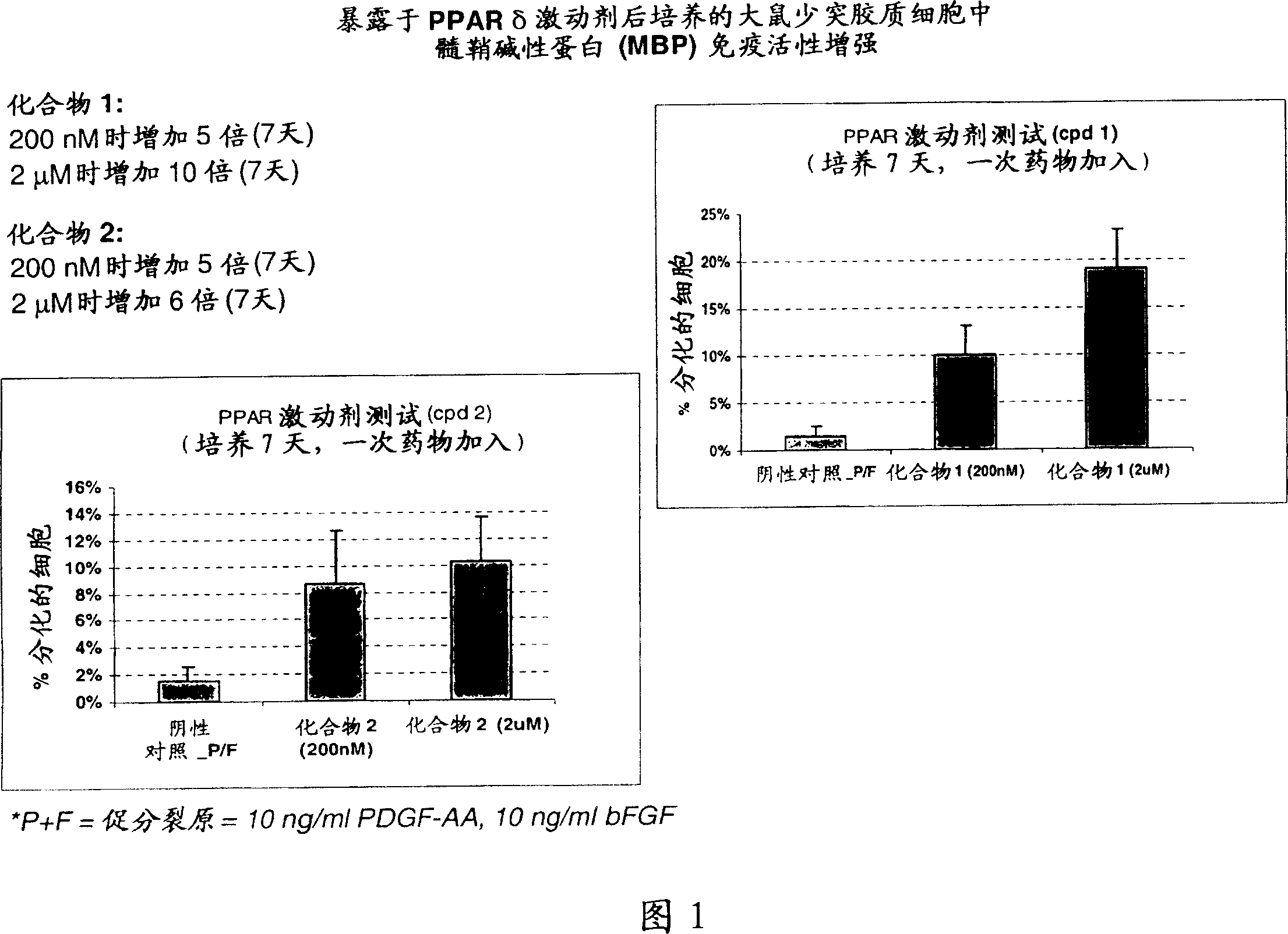
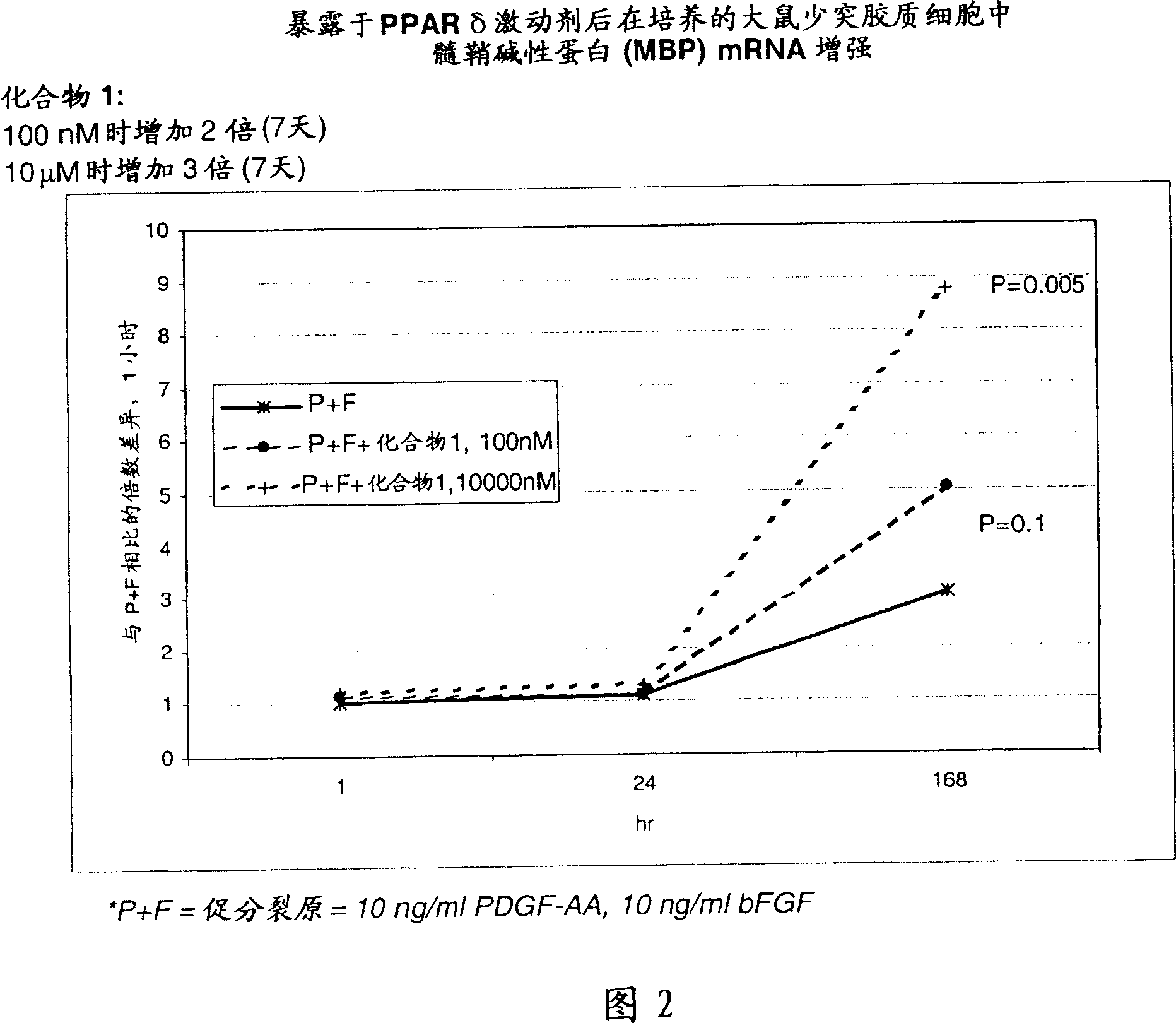
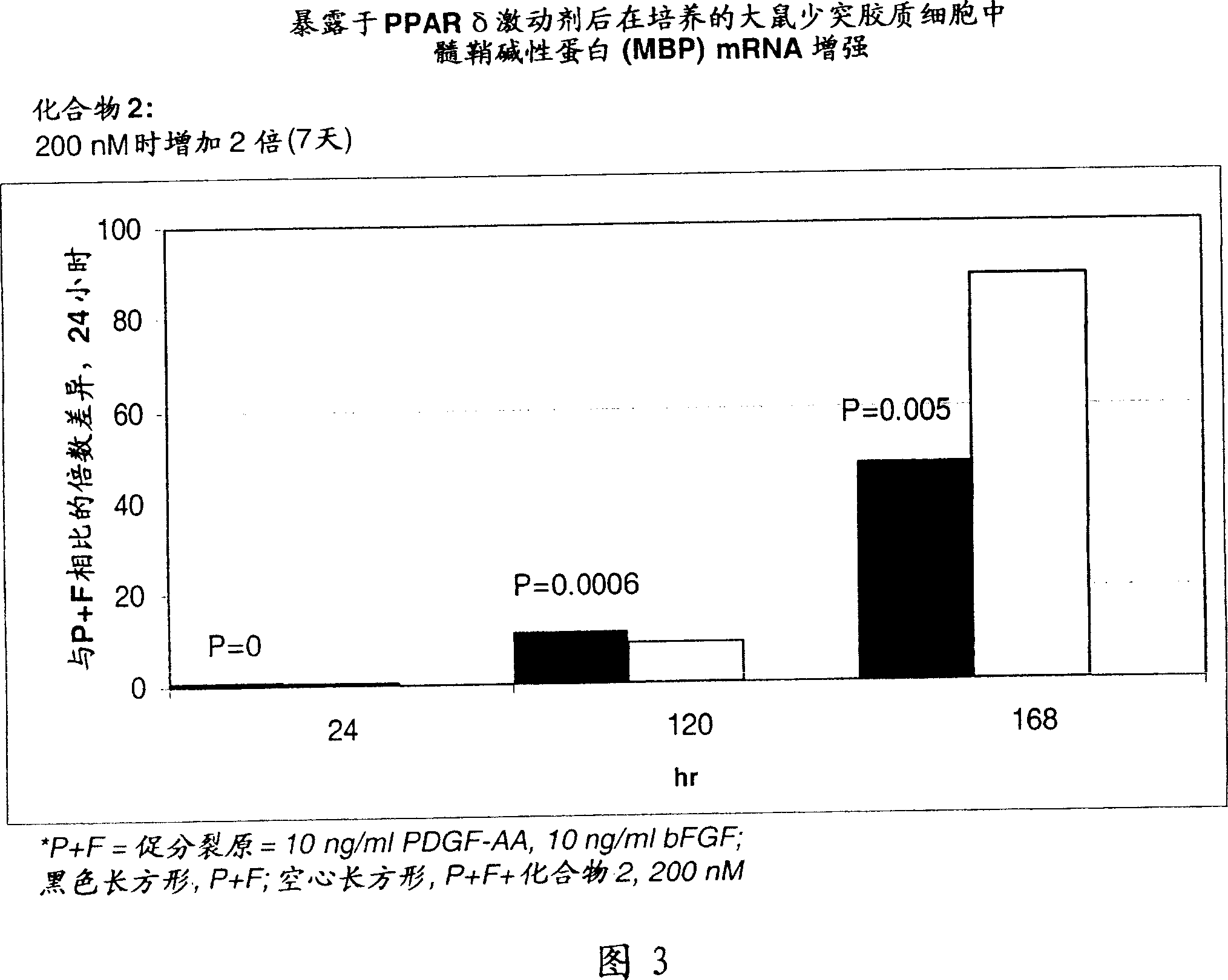
Use of peroxisome proliferator activated receptor delta agonists for the treatment of ms and other demyelinating diseases
A technology for multiple sclerosis and demyelinating diseases, applied in the field of treatment of MS and other demyelinating diseases, can solve the problem that the treatment has not been proved to be effective for humans
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] As used herein, the expression "pharmaceutically acceptable carrier" means a non-toxic solvent, dispersant, excipient, adjuvant or other compound with which the compound of the present invention is mixed to form a pharmaceutical composition, that is, capable of being administered to a patient. dosage form of the substance. An example of such a carrier is a pharmaceutically acceptable oil, which is usually used for parenteral administration.
[0022] The term "pharmaceutically acceptable salt" as used herein means that the salts of the compounds of the present invention can be used in the preparation of medicines. However, other salts may be used in the preparation of the compounds according to the invention and their pharmaceutically acceptable salts. Suitable pharmaceutically acceptable salts of the compounds of the invention include acid addition salts which can be prepared by mixing a solution of the compounds of the invention with a pharmaceutically acceptable acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com