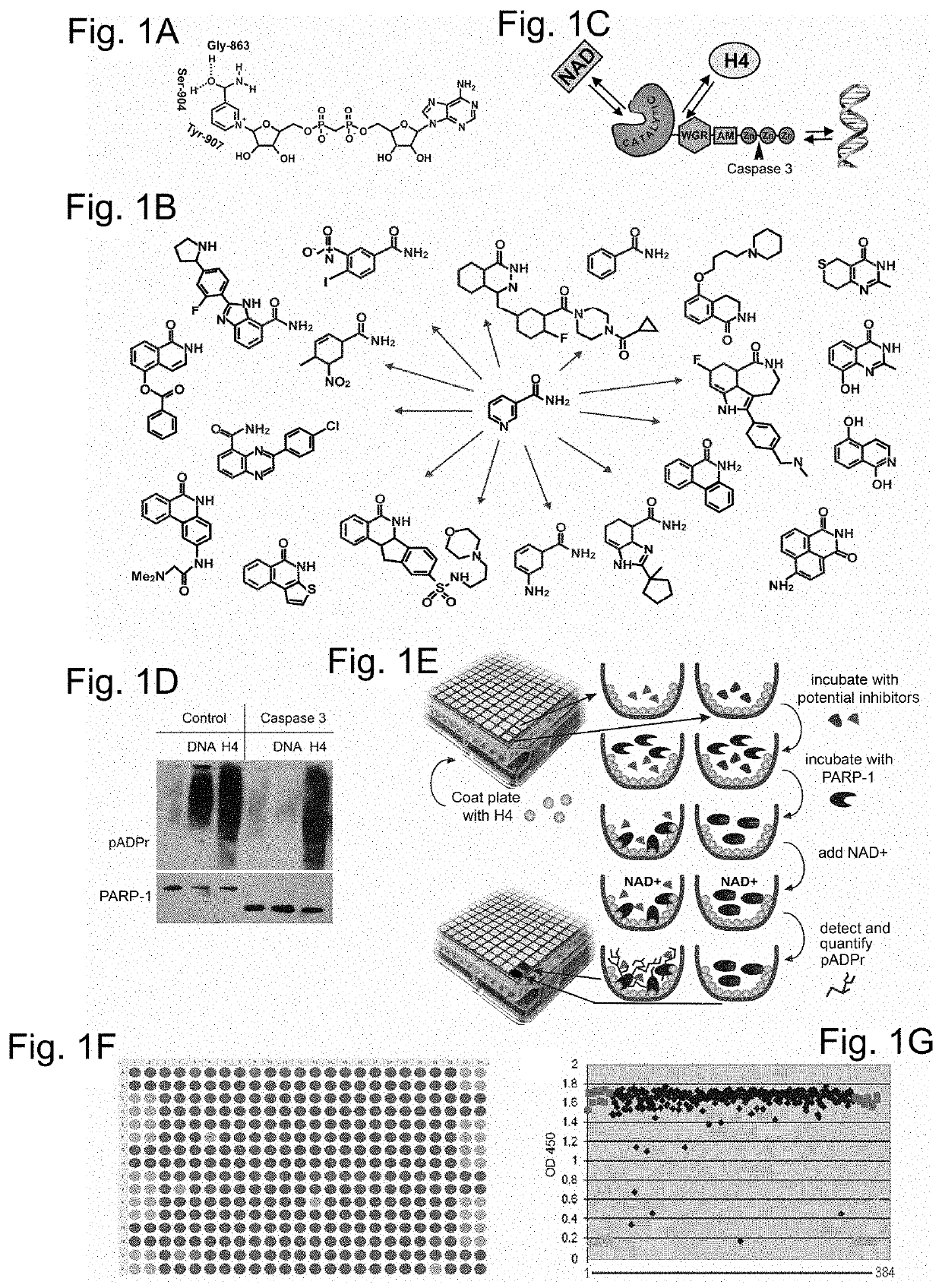
Poly(ADP-ribose) polymerase 1 inhibitors structurally unrelated to NAD
a polymerase 1 and adp-ribose technology, applied in the field of formulation chemistry, can solve the problems of obstructing the function of enzymes, difficult to completely eliminate the nad interaction with parp-1 without drastic affecting other metabolic processes, and the number of clinical studies reported setbacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
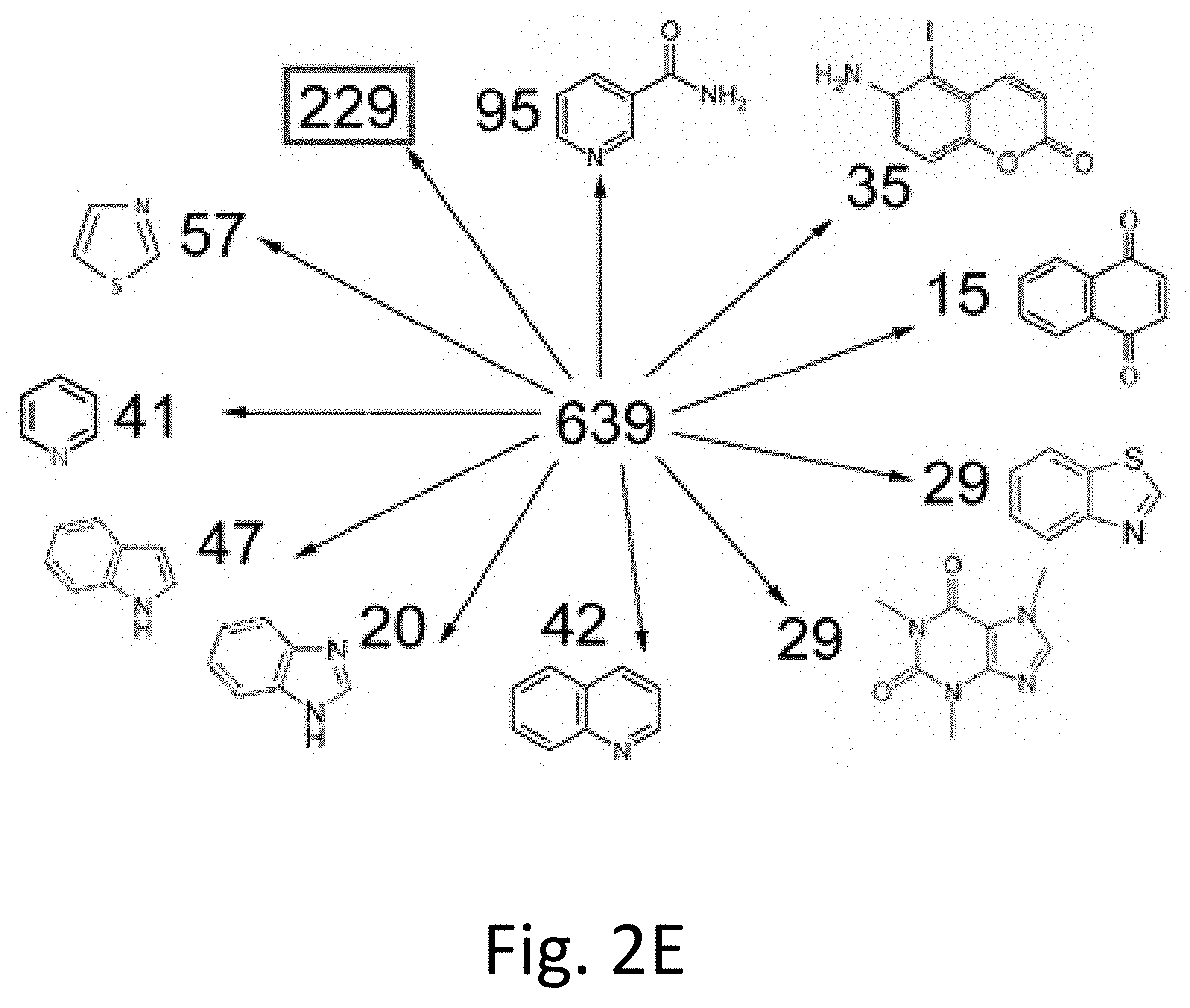
[0085]Small molecule clustering methods. Inhibitor molecules were imported as SMILES format in the software program Canvas 1.6. Binary hashed fingerprints were calculated from the 2D structure using a dendritic methodology. These generated finger prints were then used as the basis for clustering inhibitors by two methods. The first utilized hierarchical clustering with a Tanimoto similarity metric and Schrödinger cluster linkage method yielded 22 clusters by default. Alteration of the merging distance parameter in the resulting dendrogram from 0.96 to 0.9 yielded 96 clusters. The second clustering methodology involved self-organizing maps calculated according to the sum of fingerprint distances to 27 known PARP inhibitors. Molecules were parsed into a 10 by 10 grid according to this self-organizing approach, measuring similarity to known inhibitors. Twenty seven individual heat maps of the 10 by 10 grid showing distance to individual inhibitors were output for c...
example 2
PARP-1 Inhibitor Screen
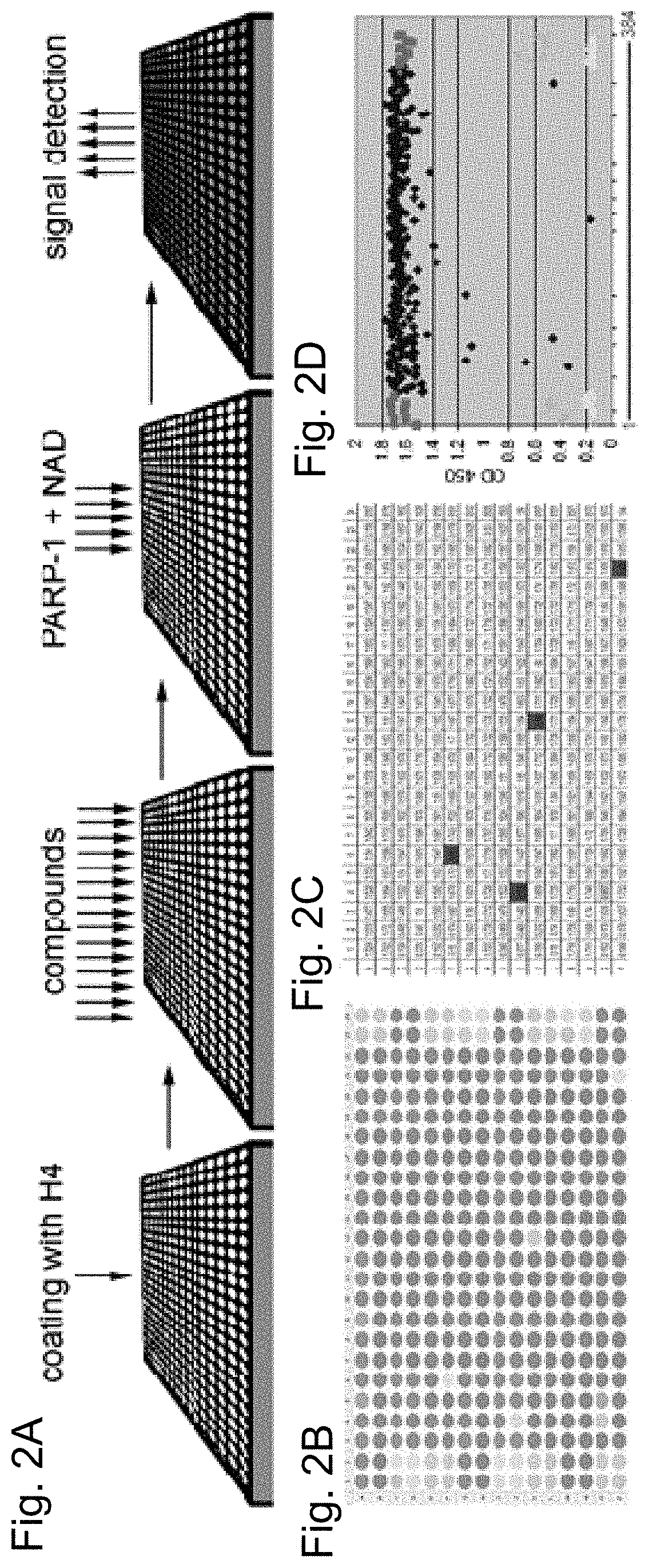
[0092]A PARP-1 activation screening assay was designed using a 384-well ELISA plate coated with histone H4 protein-activator. PARP-1 reactions were set up in each well in presence of single a small molecule compound or a positive and a negative control. Compounds that disrupt PARP-1 interaction with H4 activator or compete with NAD+ diminish or abolish accumulation of poly-(ADP)-ribose, the product of these reactions (FIG. 1E). The product of these reactions, poly-(ADP)-ribose, was quantified. Absorbance at 650 or 450 nm was used as an indicator of PARP-1 activity (FIG. 2A). As the test library for this screening assay, the ICCB Known Bioactives library of 480 compounds was used, which includes all popular PARP-1 inhibitors.
[0093]In addition to two known PARP-1 inhibitors, the pilot screening of test library identified twenty three molecules previously unknown as PARP-1 inhibitors (Table 4). Following the pilot screen, analysis of 50,000 small compounds was ca...
example 3
N-methylpiperidin / N-methylmorpholino / N-methylpyrrolidine / dipxolanyl Group of New
PARP-1 Inhibitors
[0095]Even without a common structural core molecule, the compounds could expose their epitopes in a similar way to NAD-like PARP-1 inhibitors. The software Canvas 1.6 was used to eliminate small molecules that display even minimal structural similarity to known PARP-1 inhibitors. The information about structure of small molecules was imported in SMILES format. Clustering analysis was used and based on self-organizing maps calculated as the sum of the fingerprint distances to the 27 known PARP-1 inhibitors and NAD (Table 5). Similarity was higher in closely positioned cells and decreased with distance. Molecules distributed in this matrix were compared to each known PARP-1 inhibitor and NAD. The degree of similarity in each cell was represented using heat colors. After super-exposing these maps, an area of the matrix with no similarity to known PARP-1 inhibitors and NAD was identified. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
| ring structures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com