Denaturants for sympathomimetic amine salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
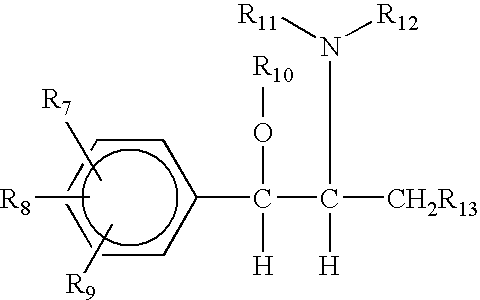
[0046] The present invention is directed to compositions comprising an acid salt of a sympathomimetic amine and at least one combination inhibitor. The compositions may also include reaction and separation inhibitors in any mixture to assure maximum protection against the use of the sympathomimetic amine-containing compositions for illegal drug manufacture.
[0047] Compositions of the Instant Invention:
examples a-h
[0048] The following non-limiting examples are for core tablets of pseudoephedrine-containing compositions. All of the ingredients are in mg per tablet. All of the tablets can be uncoated or coated using ingredients and processes known to those skilled in the art.
example a
[0049] A. Combine 30 mg pseudoephedrine HCl USP / EP, 5 mg poloxamer 407 NF, 5 mg glyceryl monostearate NF, 3 mg polyethylene oxide N-60K NF, 5 mg hydroxypropyl cellulose NF and 30 mg ethylcellulose NF and mix well. This mixture is then thermally extruded to produce a granulation.
[0050] B. Add 30 mg of aminoalkyl methacrylate copolymer E, JP with 99 mg of hydrochloric acid, Normal USP. Mix these ingredients and dry.
[0051] C. Delump 2 mg of silicon dioxide NF / EP, 30 mg lactose NF, 30 mg fructose NF and 5 mg crospovidone NF.
[0052] D. Combine the material produced from step B with the granulation of step A and size through an appropriate mill.
[0053] E. Add the delumped material to the sized material and blend until uniform.
[0054] F. Sift 2.5 mg stearic acid NF and 0.5 mg magnesium stearate NF into the blend produced in step E.
[0055] G. Compress the resultant blend on a standard press to the desired weight and thickness.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
| water insoluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com