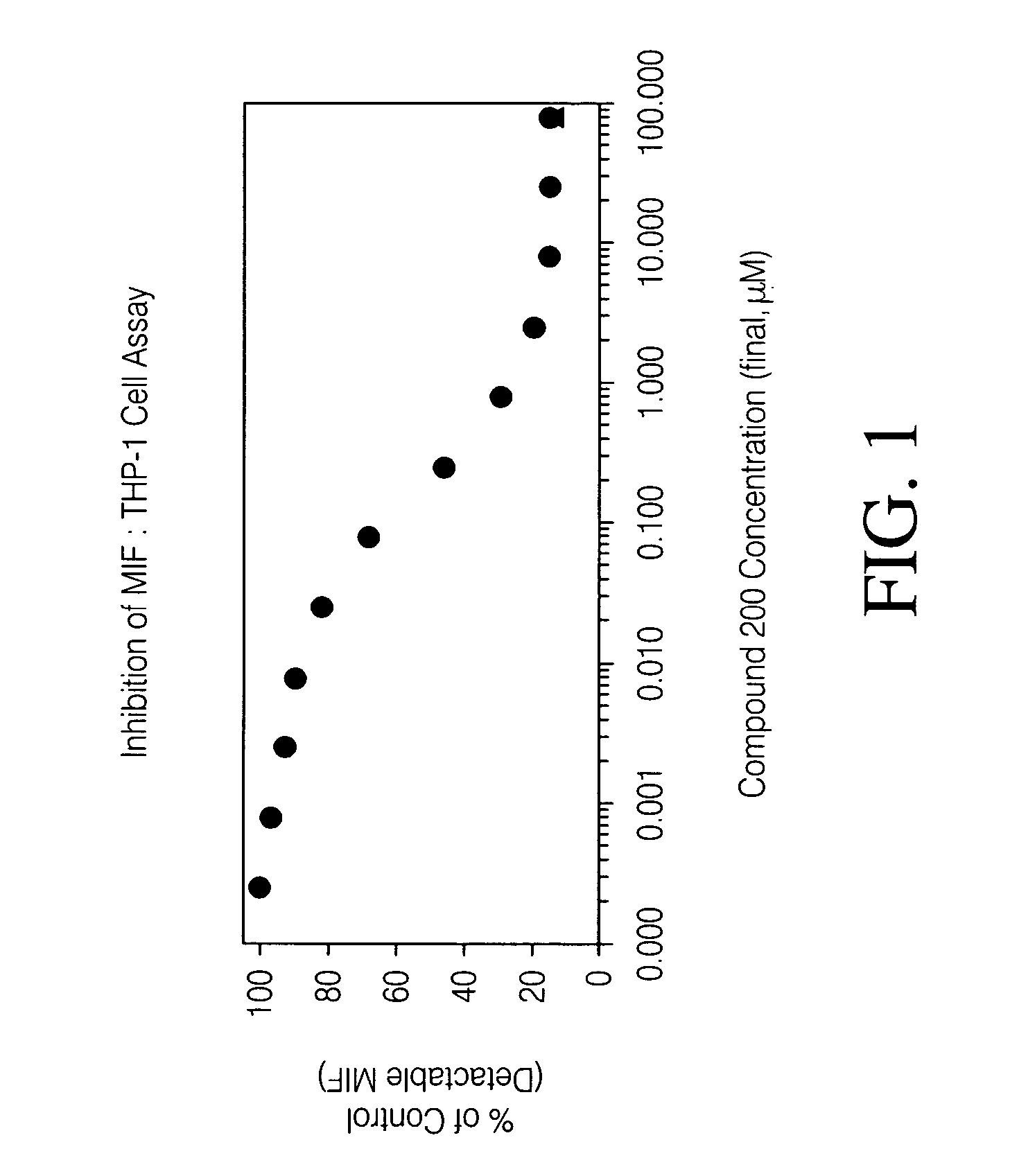
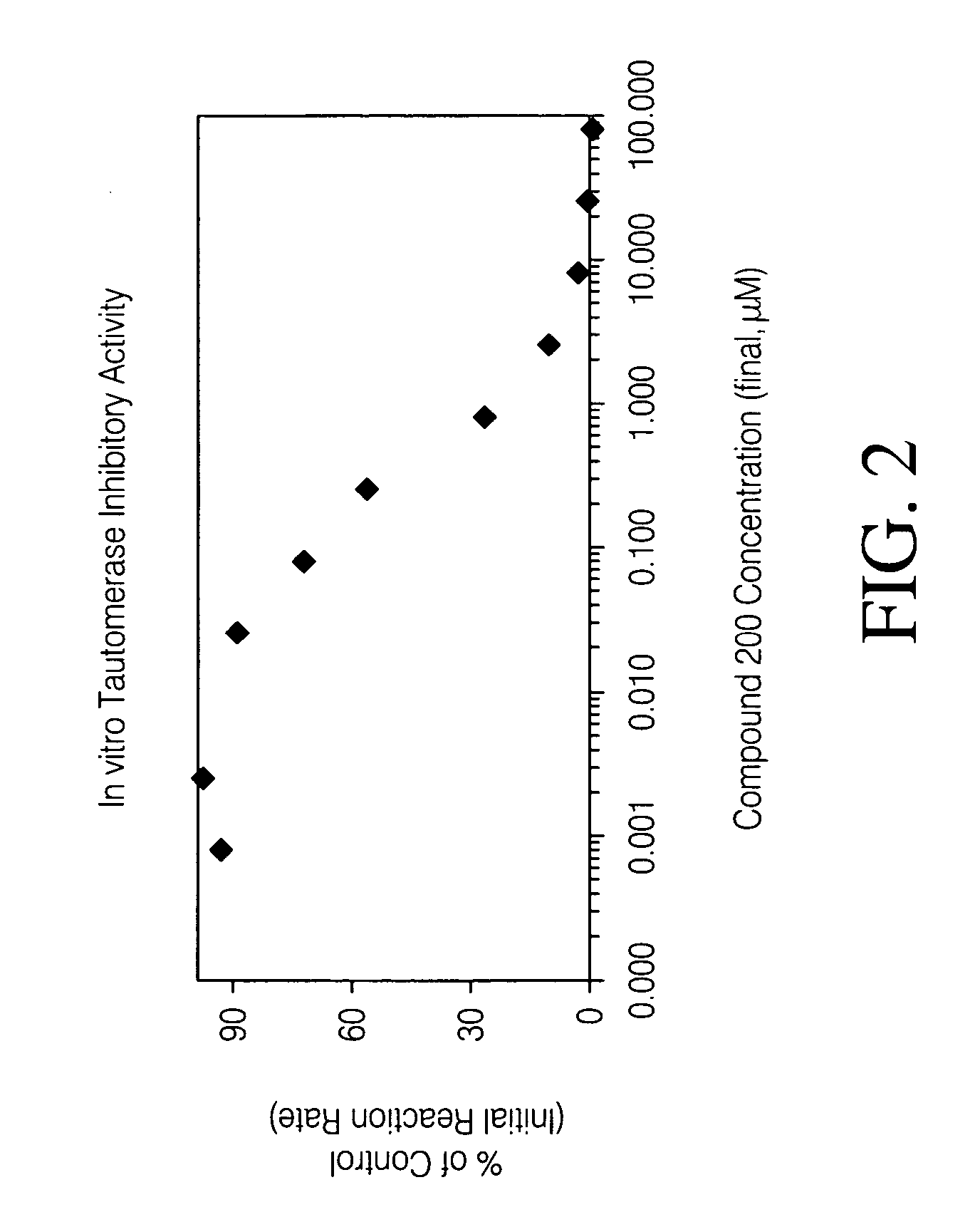
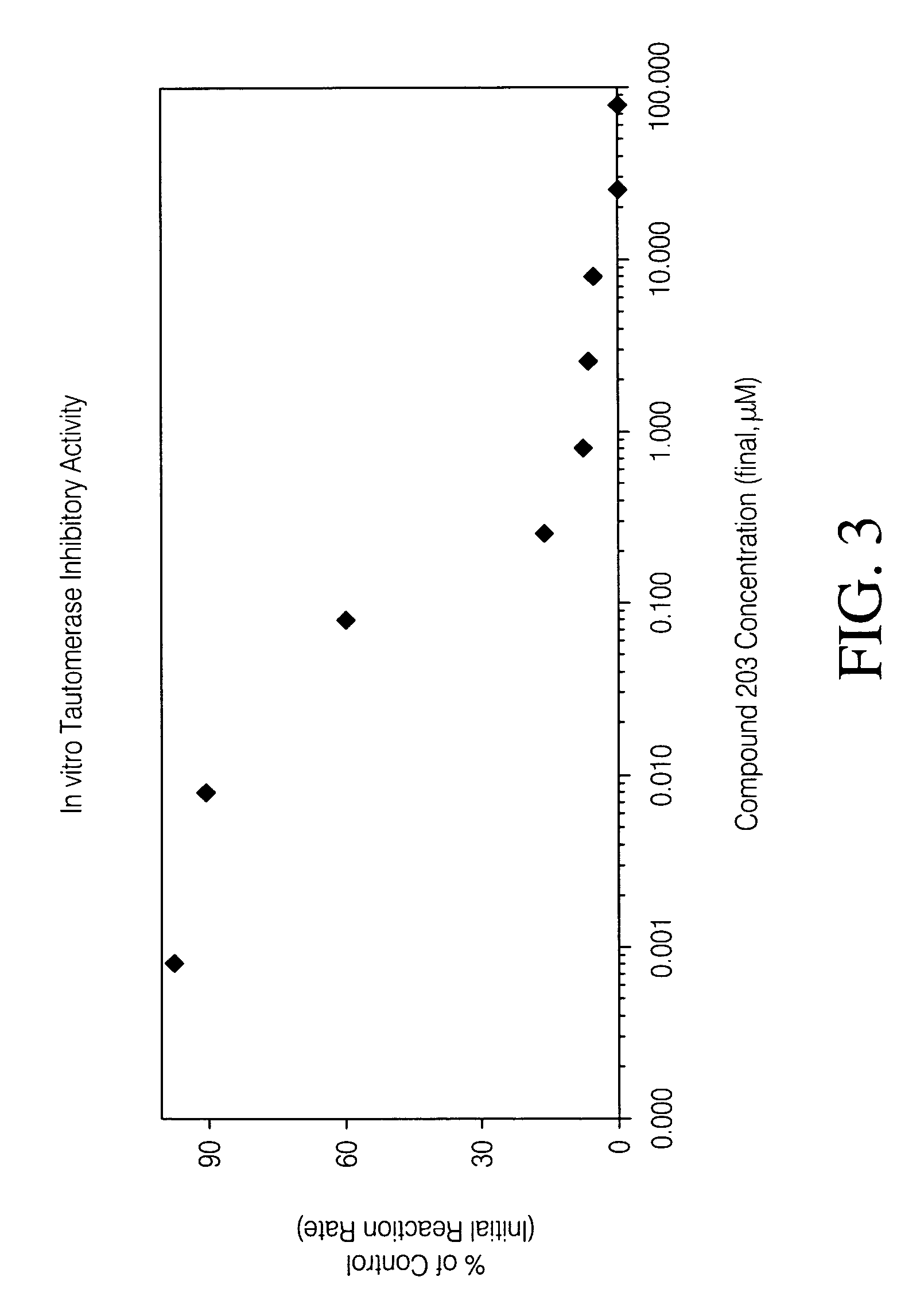
Inhibitors of macrophage migration inhibitory factor and methods for identifying the same
a macrophage migration and inhibitory factor technology, applied in the direction of antibacterial agents, immunological disorders, metabolism disorders, etc., can solve the problems of limited ability to evaluate the inhibition of mif to its cell surface receptor, significant toxicity, and toxicity of inhibitors that act intracellularly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0175] Macrophage Migration Assay
[0176] Macrophage migration is measured by using the agarose droplet assay and capillary method as described by Harrington and Stastny et al., J. Immunol. 110(3):752-759, 1973. Briefly, macrophage-containing samples are added to hematocrit tubes, 75 mm long with a 1.2 mm inner diameter. The tubes are heat sealed and centrifuged at 100×G for 3 minutes, cut at the cell-fluid interface and imbedded in a drop of silicone grease in Sykes-Moore culture chambers. The culture chambers contain either a control protein (BSA) or samples. Migration areas are determined after 24 and 48 hours of incubation at 37° C. by tracing a projected image of the macrophage fans and measuring the areas of the migration by planimetry.
[0177] Alternatively, each well of a 96-well plate is pre-coated with one microliter of liquid 0.8% (w / v) Sea Plaque Agarose in water dispensed onto the middle of each well. The plate is then warmed gently on a light box until the agarose drops ...
example 2
[0219] Inhibitors of MIF of certain embodiments may be prepared according to the following reaction schemes. Each of these MIF inhibitors belongs to one of the classes of compounds described above. The variables R1, R2, R3, R4, Z, and n are as defined above.
[0220] General Methods for the Synthesis of the Compounds of the Inventions
[0221] The compounds were synthesized starting from substituted or unsubstituted isatoic anhydrides. The strategies to introduce R2 and / or R3 groups into the compounds of structures (1a) and (1b) described above involved preparation of substituted isatoic anhydrides as precursor compounds from substituted anthranilic acids. The substituted anthranilic acids were prepared from substituted nitrobenzoic acids. In some cases, the nitro benzoic acids were obtained by nitration of appropriate benzoic acid, as shown in Reaction Scheme 1, however, any suitable method for preparation of nitrobenzoic acids may be employed.
[0222] Two different methods were employ...
example 3
[0231] The following describes the synthesis of a library of compounds of general structure 1′(a) and 1′(b) as depicted below. Compounds including “M” in the designation incorporate the CN moiety. Numerical designations in Table 1 are as given below.
[0232] In compounds that have designations including “+i”, R,13 is a substituent on the oxygen atom of the quinolone group rather than the nitrogen atom, i.e., a compound of structure 1′(b), as depicted below. The designation “i” appears elsewhere in the preferred embodiments, and refers to a substituent on the oxygen atom of the quinolone group rather than the nitrogen atom.
Numerical Designations for R11 Functional Groups
[0233]
HydrogenMethylChlorine123
Numerical Designations for R12 Functional Groups
[0234]
Numerical Designations for R13 Functional Groups
[0235]
[0236] The numerical designations of the MIF inhibitors prepared are provided in Table 1.
TABLE 1R13 = 1R13 = 2R13 = 4R13 = 5R13 = 6Methyl1M111M121M131M141M15 + i1M211M221M231...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com