Novel TNF receptor death domain ligand proteins and inhibitors of ligand binding
a technology of tnf receptor and ligand protein, which is applied in the direction of peptide/protein ingredients, dna/rna fragmentation, fungi, etc., can solve the problems that the ligands which bind to the tnf-r intracellular domain have yet to be identified, so as to prevent or ameliorate an inflammatory condition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of TNF-R Death Domain Ligand Protein Encoding Polynucleotide
[0183]A yeast genetic selection method, the “interaction trap” [Gyuris et al. Cell 75:791-803, 1993, which is incorporated herein by reference], was used to screen W138 cell cDNA libraries (preparation, see below) for proteins that interact with the death domain of the P55 type 1 TNF receptor (TNF-R1-DD). A polynucleotide encoding amino acids 326 to 413 of the P55 type TNF receptor, TNF-R1-DD, was obtained via the polymerase chain reaction (PCR) using a grafting method. This TNF-R1-DD DNA was then cloned into pEG202 by BamHI and SalI sites, generating the bait plasmid, pEG202-TNF-R1-DD. This plasmid contains the HIS3 selectable marker, and expression of the bait, the LexA-TNF-R1-DD fusion protein, is from the strong constitutive ADH1 promoter. To create the reporter strain carrying the bait protein, yeast strain EGY48, containing the reporter sequence LexAop-Leu2 in place of the chromosomal LEU2, was transformed wit...
example 2
Expression of the TNF-R1-DD Ligand Protein
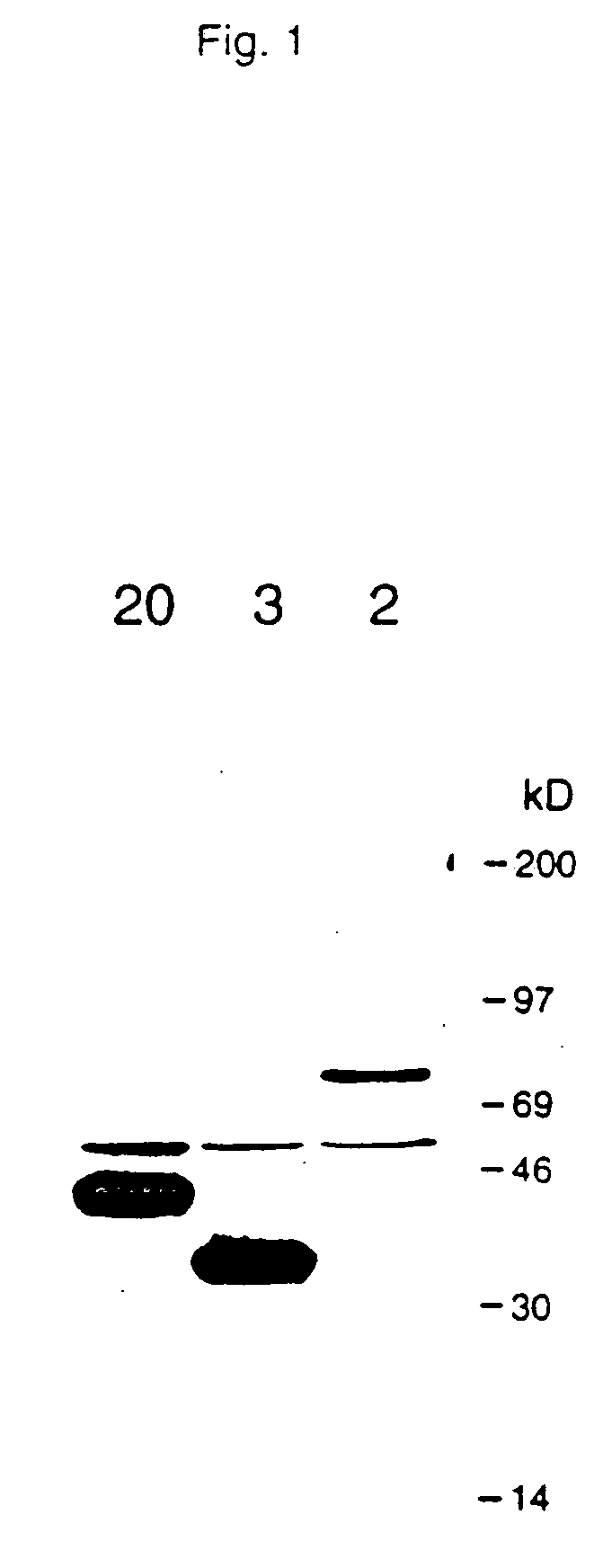
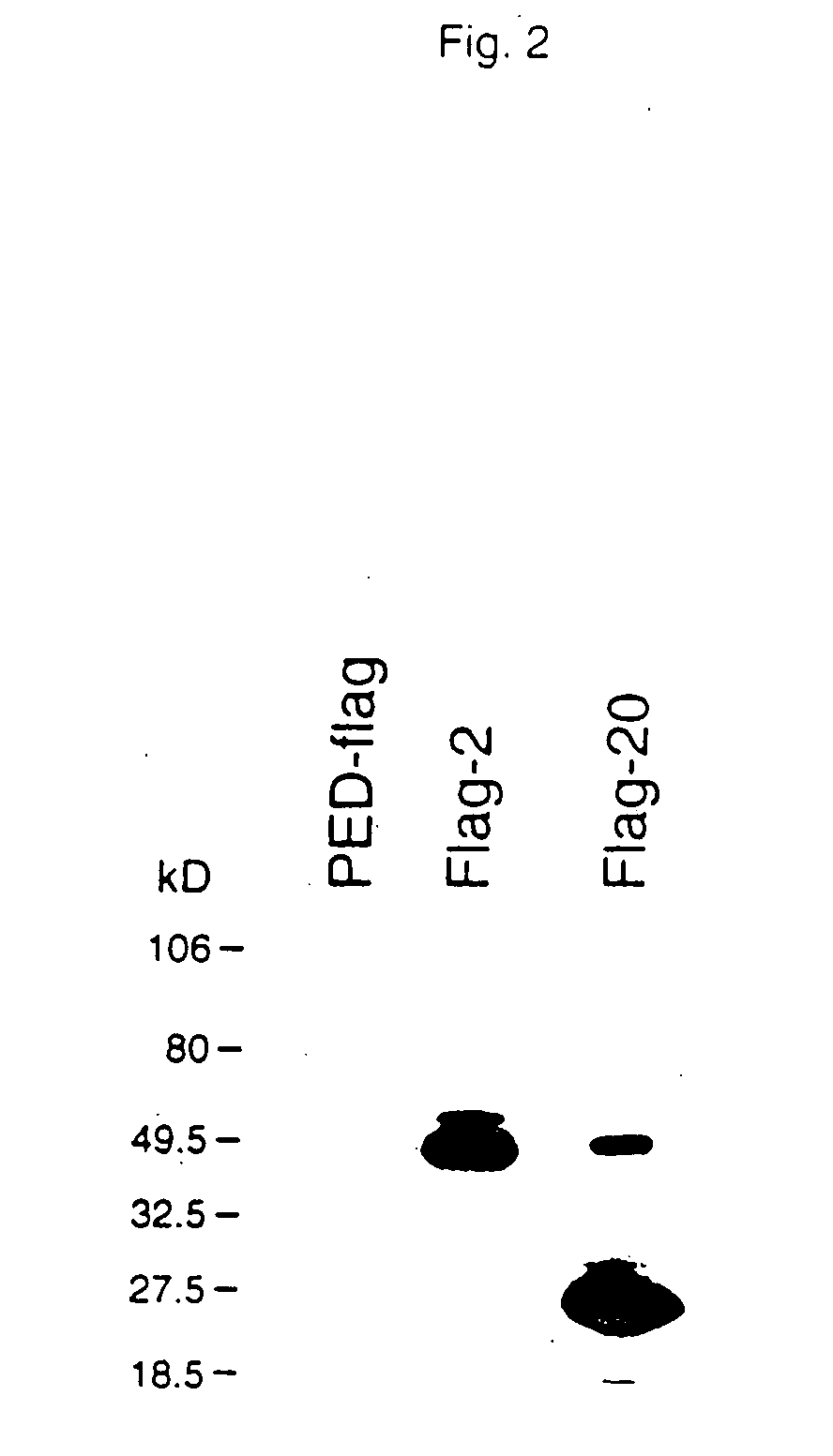
[0191]cDNAs encoding TNF-R intracellular ligand proteins were released from the pJG4-5 vector with the appropriate restriction enzymes. For example, EcoRI and XhoI or NotI and XhoI were used to release cDNA from clone 2DD and clone 20DD. Where the restriction sites were also present in the internal sequence of the cDNA, PCR was performed to obtain the cDNA. For example, the cDNA fragment encoding “clone 3DD” was obtained through PCR due to the presence of an internal XhoI site. These cDNAs were then cloned into various expression vectors. These included pGEX (Pharmacia) or pMAL (New England Biolabs) for expression as a GST (Glutathione-S-transferase) or MBP (maltose binding protein) fusion protein in E. coli, a pED-based vector for mammalian expression, and pVL or pBlueBacHis (Invitrogen) for baculovirus / insect expression. For the immunodetection of TNF-R intracellular ligand expression in mammalian cells, an epitope sequence, “Flag,” was in...
example 3
Assays of TNF-R Death Domain Binding
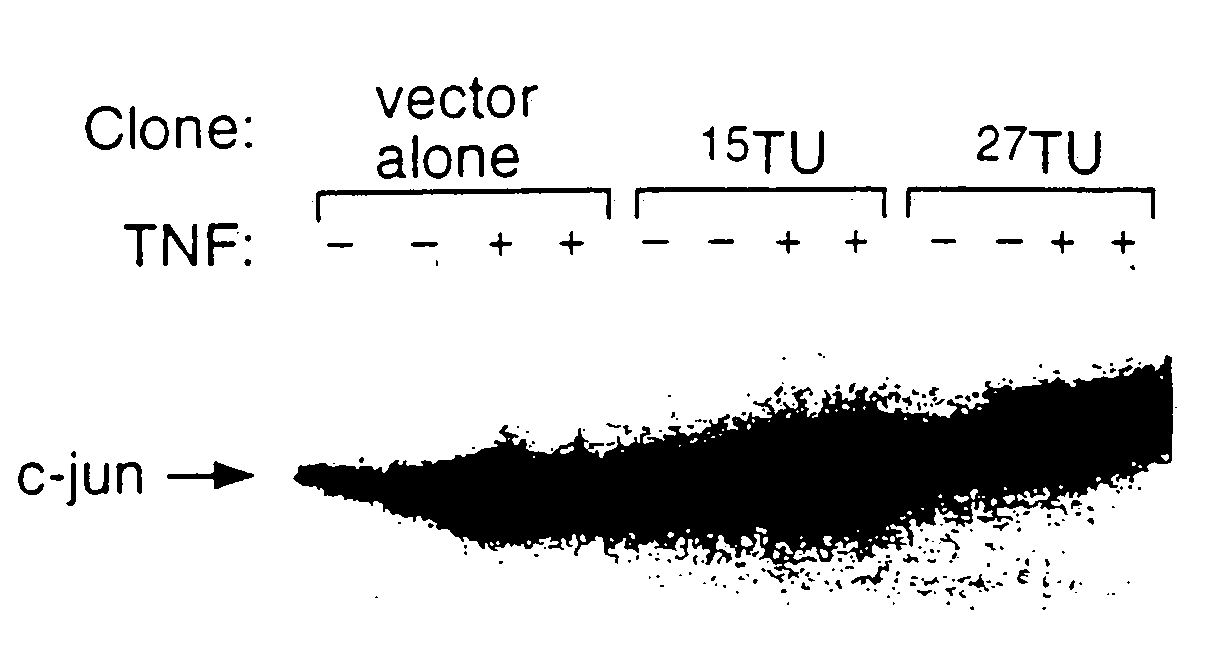
[0193]Two different methods were used to assay for TNF-R1-DD ligand protein activity. The first assay measures binding in the yeast strain in “interaction trap,” the system used here to screen for TNF-R1-DD interacting proteins. In this system, the expression of reporter genes from both LexAop-Leu2 and LexAop-LacZ relies on the interaction between the bait protein, in this case TNF-R1DD, and the prey, the TNF-R intracellular ligand. Thus, one can measure the strength of the interaction by the level of Leu2 or LacZ expression. The most simple method is to measure the activity of the LacZ encoded protein, β-galactosidase. This activity can be judged by the degree of blueness on the X-Gal containing medium or filter. For the quantitative measurement of β-galactosidase activity, standard assays can be found in “Methods in Yeast Genetics” Cold Spring Harbor, N.Y., 1990 (by Rose, M.D., Winston, F., and Hieter, P.).
[0194]The second assay for measuring bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com