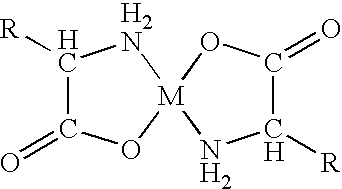
Mouth hygienic composition for the treatment of halitosis
a technology for halitosis and composition, applied in the field of mouth hygienic composition for the treatment of halitosis, can solve the problems of affecting the treatment effect, and affecting the treatment effect, and achieves the effects of reducing the number of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
It was initially decided to analyse various zinc-containing compounds for their ability to reduce and / or eliminate halitosis. The zinc-compounds are listed in Table 1 below.
More than 16 different experiments were carried out with these compounds. With the exception of the zinc amino acid chelate TF (tastefree) 10% Zn, product no. 3463, (Albion Laboratories, Inc., Clearfield, Utah 84015, USA), zinc acetate, zinc chloride, zinc citrate, ZnSO.sub.4 (H.sub.2 O).sub.6 and zinc gluconate all had very poor organoleptic qualities, particularly a pronounced metallic taste, effectively preventing these compounds from being used commercially as an effective inhibitor of halitosis. It was not possible to find a way in which to reduce or eliminate this metallic taste.
It was generally found that a treatment for halitosis was most effective when a high concentration of Zn was employed and when the zinc compound had a high degree of solubility.
The results obtained by using the zinc amino acid chela...
example 2
The below Table shows the ingredients used for preparing a lozenge, which was subsequently used in a "cysteine challenge test" in order to analyse its effectiveness in inhibiting halitosis. The zinc amino acid chelate was initially granulated with P.V.P. in isopropanol according to standard procedures. Xylitol was dried at 35.degree. C. and added to the granulate along with the other ingredients in the amounts indicated in the Table. The lozenge produced in accordance with standard preparation techniques had a weight of approximately 0.5 g.
The lozenge was used in a "cysteine challenge test", and volatile sulphur compounds were detected by using a Halimeter, as described in the below Example 3.
example 3
The use of a Halimeter for testing the mouth hygienic effects of the lozenge having the composition as described in Example 2 showed that the lozenge is remarkably effective in reducing and inhibiting halitosis.
After initial measurement of a VSC base-line value, a mouthwash or rinse was performed with a cysteine solution (6 mM, pH=7.2), and the VSC value was again measured. After approximately 30-40 minutes the lozenge was administered to the oral cavity, and the VSC value was measured immediately after this administration.
Repeated mouthwashes at suitable time points after the administration, as indicated in Table 3 and FIG. 1, facilitated the analysis of the effect of the lozenge as an effective inhibitor of Halitosis.
The results show that an average reduction of VSC production of almost 50% was observed three hours after administration of the lozenge. Importantly however, immediately after intake of the lozenge, a reduction in VSC of more than 80% was measured. One hour after inta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| atomic weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com