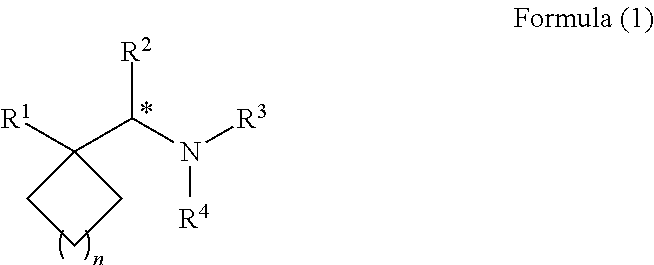
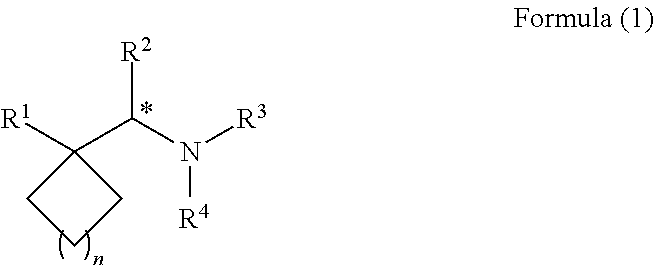
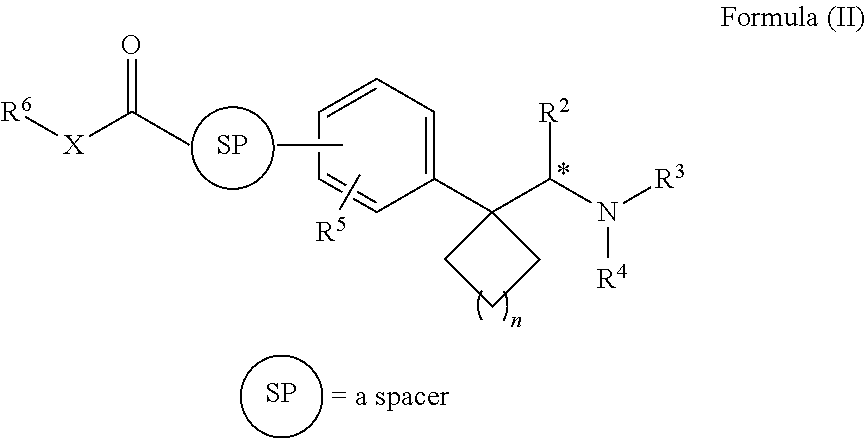
Synthesis, methods of using, and compositions of cycloalkylmethylamines
a technology of cycloalkylmethylamine and synthesis method, which is applied in the field of cycloalkylmethylamines, synthesis of cycloalkylmethylamines, can solve the problems of limited advances in the pharmacotherapy of this condition, increased risk of alzheimer's-type dementia, and increased risk of certain types of cancer, etc., to achieve favorable metabolic, pharmacokinetic and pharmacological profiles, and high pharmacokinetic profiles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for Synthesis of 49a-d (Scheme 7)
[0189]The phenylcyclobutanenitriles 49a-d were prepared according to the protocol reported by Butler and Polatz (J. Org. Chem. 1971, 36, 1308). To a stirred suspension of sodium hydride (NaH) (0.1 mole, 2.4 g) in 25 mL of anhydrous tetrahydrofuran (THF) under nitrogen atmosphere at ice-bath temperature was dropwise added a solution of 1,3-dibromopropane (11.10 g, 0.055 mole) and appropriate benzylnitrile 47a-d (0.05 mole) in 50 mL of THF. The resulting mixture was slowly warmed to room temperature and continued stirring overnight (12 hours) at room temperature. The progress of the reaction was monitored by thin layer chromatography (TLC). The reaction was poured onto crushed ice (200 g) and then, extracted with ethyl acetate (100 mL×3). The combined extract was washed with water (100 mL×2), dried over magnesium sulfate (MgSO4) and evaporated under reduced pressure to give the corresponding phenylcyclobutanenitriles 49a-d which were ...
example 2
General Procedure for Synthesis of 52a-d (Scheme 7)
[0194]To a stirred solution of 2M isobutyl magnesium bromide in diethyl ether (0.04 mole, 20 mL) under nitrogen atmosphere at room temperature was dropwise added a solution of appropriate cyclobutanenitrile 49a-d (0.025 mole) in 20 mL of anhydrous THF or toluene. The resulting mixture was refluxed for 18-24 hours. The progress of the reaction was monitored by TLC. In a separate round bottom flask 25 mL of anhydrous isopropanol was taken and sodium borohydride (3.00 g, 0.08 mole) was added to isopropanol portion-wise at room temperature. After having stirred for 10 minutes, the Grignard adduct from the reaction flask was directly added into the stirred solution sodium borohydride in isopropanol under nitrogen atmosphere. The resulting mixture was refluxed for 12 to 18 hours. The progress of the reaction was monitored by TLC. The reaction mixture was slowly poured onto a mixture of crushed ice (200 g) and sodium bicarbonate (5.00 g). ...
example 3
General Procedure for Synthesis of 54a-e (Scheme 7)
[0200]To a stirred suspension of cesium carbonate (Cs2CO3) (1.30 g, 0.004 Mole) in 20 mL of anhydrous N,N-dimethylformamide (DMF) under nitrogen atmosphere at room temperature was added tetrabutylammonium iodide (TBAI) (1.47 g, 0.004 mole) followed by appropriate cyclobutane amine 52a-d (0.0035 mole). After having stirred for 30 minutes, a solution of appropriate bromoalkylcarboxylic esters 53 in 5 mL of DMF was introduced into the reaction mixture dropwise. The resulting mixture was stirred for 18-24 hours and the progress of the reaction was monitored by TLC. The reaction mixture was diluted with ethyl acetate (50 mL), filtered through a CELITE® pad and washed the CELITE® pad with ethyl acetate (15 mL×3). The combined filtrate was washed with brine (50 mL), water (50 mL), dried over sodium sulfate (Na2SO4) and evaporated. The residue was purified by silica gel column chromatography technique using 0-50% gradient of ethyl acetate a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com