CGRP receptor antagonist
a technology of cgrp receptor and antagonist, which is applied in the direction of peptides, drug compositions, cardiovascular disorders, etc., can solve the problems of lack of molecular evidence and cgrp2 receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
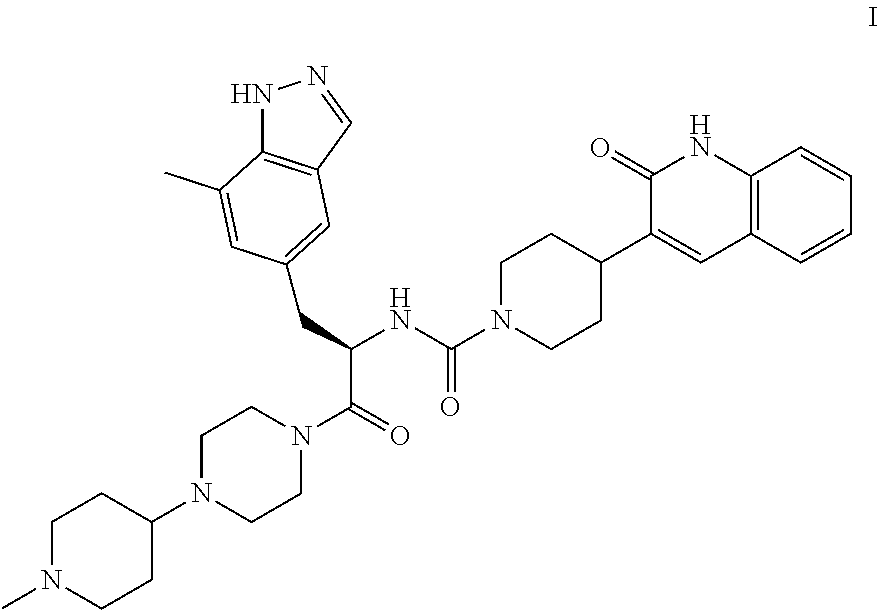
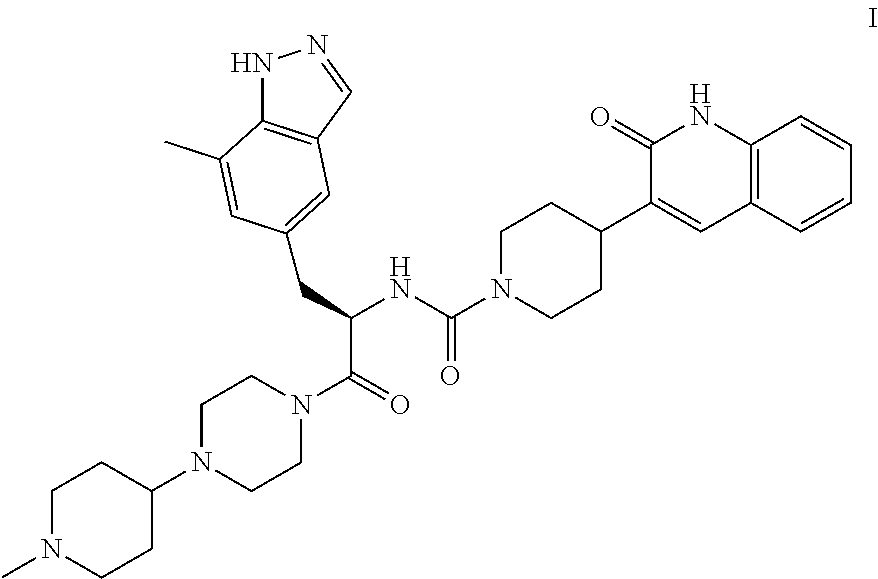
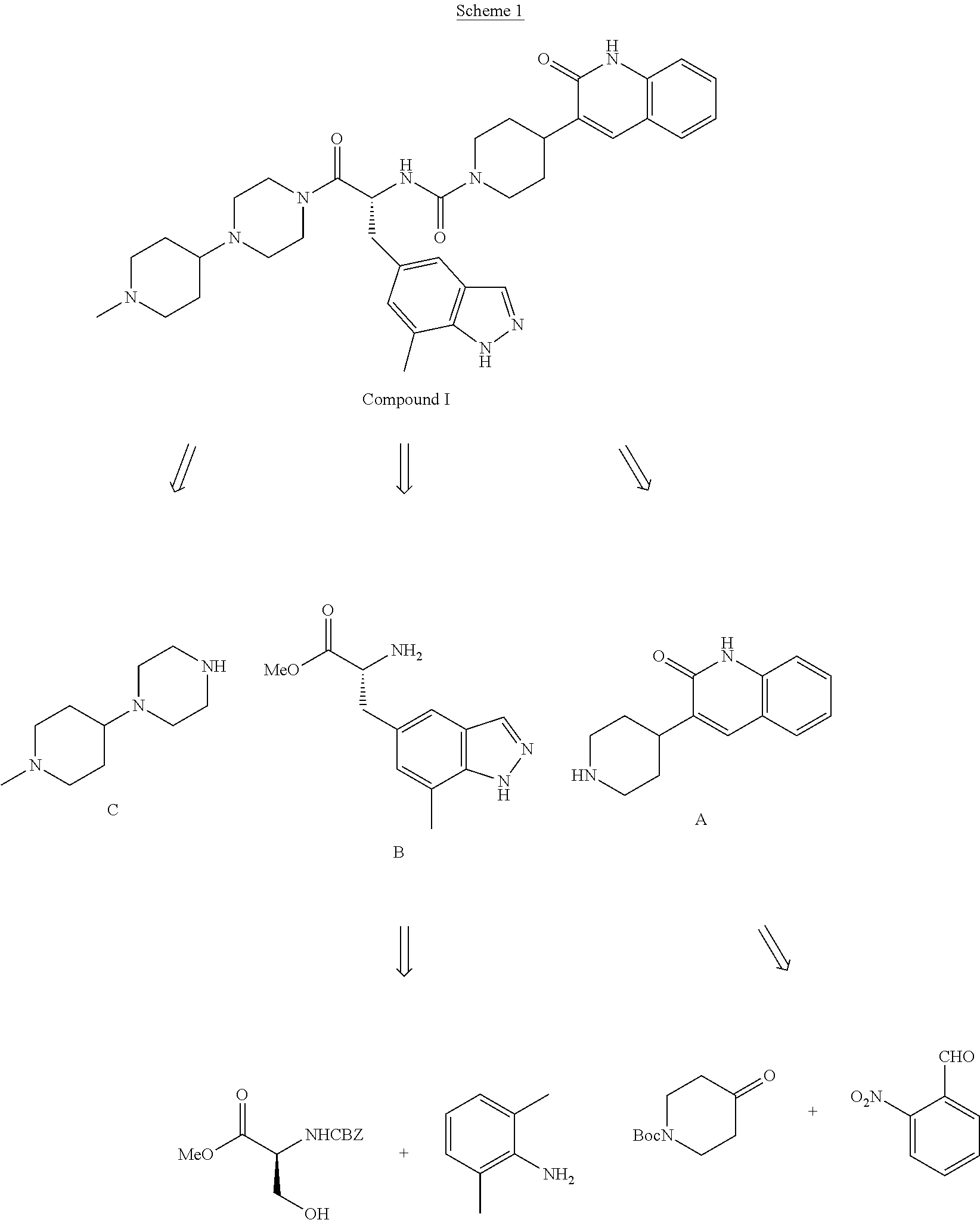
[0009]The invention encompasses (R)—N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)piperazin-1-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (Compound I or the compound of formula I) and pharmaceutical compositions and methods for modulating CGRP and treating patients with medical conditions associated with aberrant levels of CGRP or CGRP receptor signaling.
[0010]
[0011]The invention includes all pharmaceutically acceptable salt forms of the compounds. Pharmaceutically acceptable salts are those in which the counter ions do not contribute significantly to the physiological activity or toxicity of the compounds and as such function as pharmacological equivalents. These salts can be made according to common organic techniques employing commercially available reagents. Some anionic salt forms include acetate, acistrate, besylate, bromide, chloride, citrate, fumarate, glucouronate, hydrobromide, hydrochloride, hydroiodide, iodide, lactate,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com