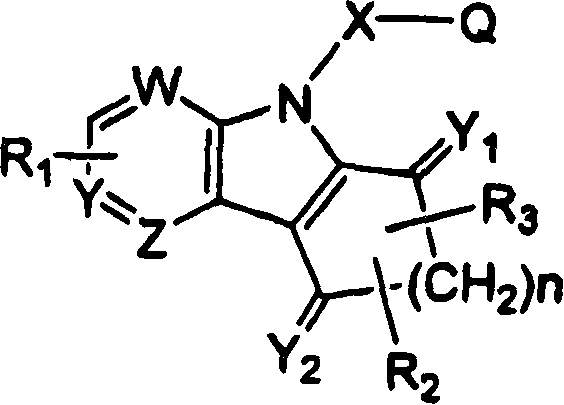
Ophthalmic compositions for treating ocular hypertension
A technology of compounds and mixtures, applied in the field of ophthalmic compositions for treating ocular hypertension, can solve problems such as unsatisfactory distribution of effects and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
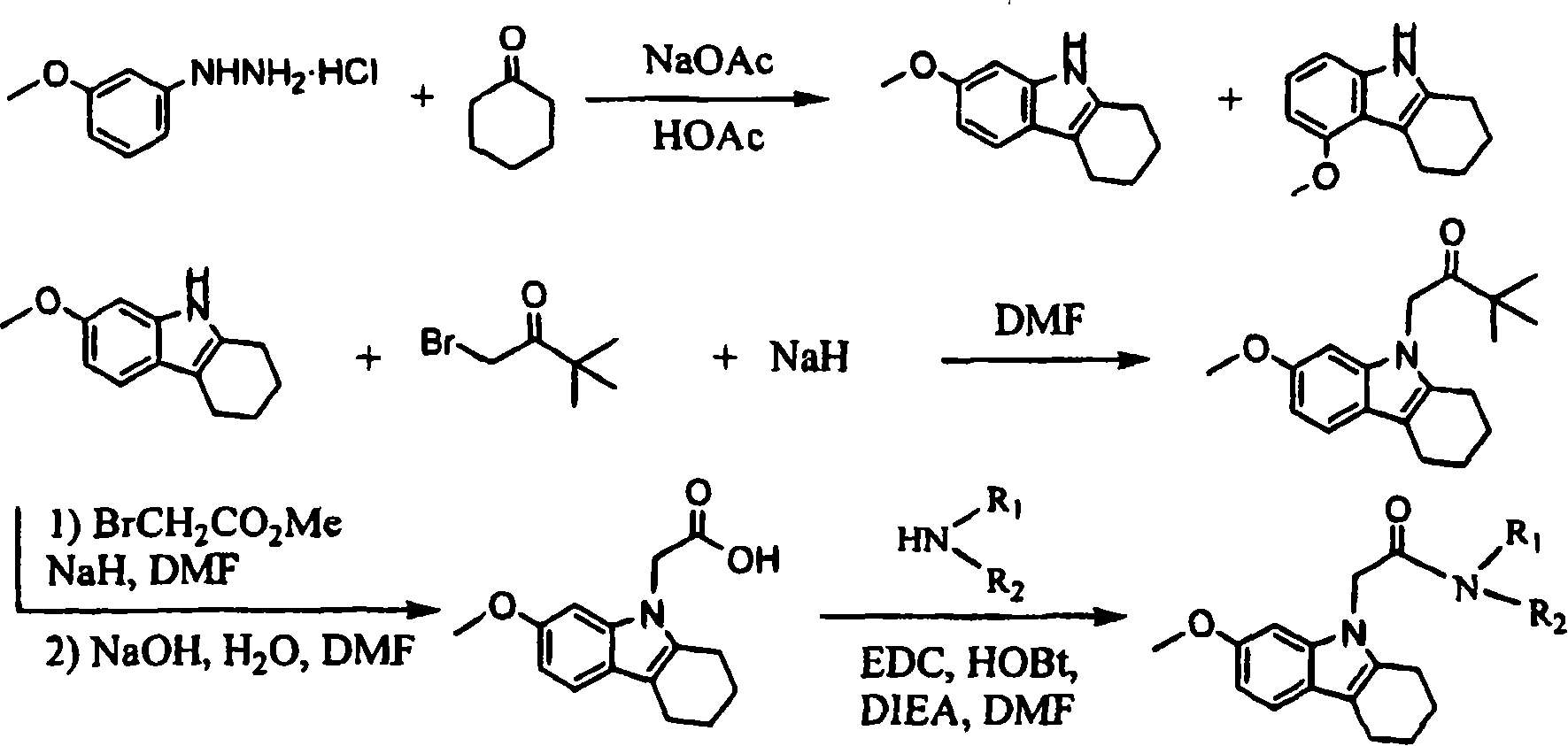
Embodiment 1
[0148]
[0149] 1-(7-methoxy-1,2,3,4-tetrahydro-9H-carbazol-9-yl)-3,3-dimethylbutan-2-one
[0150] Step A. 7-Methoxy-2,3,4,9-tetrahydro-1H-carbazole
[0151] A mixture of 4.04 g of 3-methoxyphenylhydrazine hydrochloride, 2.27 g of cyclohexanone and 1.90 g of sodium acetate was refluxed in 16 ml of acetic acid for 4 hours under a nitrogen atmosphere. The solvent was removed under reduced pressure. The residue was partitioned between water and EtOAc. The combined EtOAc extracts were washed with 0.1N HCl, 5% NaHCO 3 and saturated brine, washed with anhydrous Na 2 SO 4 Drying and evaporation gave crude product. The latter was repeatedly purified on silica gel using 15-25% EtOAc / hexanes to afford two isomeric products. The slow eluting isomer is the title compound. 1 H NMR (CDCI3, 500MHz) δ7.57(brs, 1NH), 7.35(d, 8.5Hz, 1H), 6.84(d, 2.1Hz, 1H), 6.77(dd, 2.1 & 8.5Hz, 1H), 3.86( s, 3H), 2.67-2.74 (m, 4H), 1.85-1.95 (m, 4H). LC-MS: 3.60 min. (m / Z = 202.2). The faster elutin...
Embodiment 2
[0155]
[0156] N,N-Dibutyl-2-(7-methoxy-1,2,3,4-tetrahydro-9H-carbazol-9-yl)acetamide
[0157] Step A. (7-Methoxy-1,2,3,4-tetrahydro-9H-carbazol-9-yl)acetic acid
[0158] To a solution of 0.25 g of 7-methoxy-2,3,4,9-tetrahydro-1H-carbazole in 10 mL of anhydrous DMF from step A of Example 1 was added 150 mg of NaH (60% oil dispersion). After 10 minutes, 0.21 g of methyl bromoacetate was added and the resulting mixture was stirred at room temperature for 5 hours. 1 mL each of water and 5N NaOH was carefully added to the reaction mixture. After stirring overnight at room temperature, the solvent was removed under reduced pressure. Workup of the residue with water and ether afforded acidic fractions containing the title compound.1 H NMR (CDCl 3 , 500MHz) δ7.37(d, 8.5Hz, 1H), 6.79(dd, 2.3 & 8.6Hz, 1H), 6.70(d, 2.3Hz, 1H), 4.74(s, 2H), 3.87(s, 3H) , 2.70-2.72(m, 2H), 2.63-2.66(m, 2H), 1.93-1.98(m, 2H), 1.84-1.89(m, 2H).LC-MS: 3.29min.(m / Z=260.2 ).
[0159] Step B. N,N-Dibu...
Embodiment 3-17
[0162]
[0163] Examples 3-17 in Table 1 were prepared using the same method as described in Example 3, Step B, from the appropriate amine. The preparation of the amines used in Examples 13-16 has been described in WO2004 / 04354, which is incorporated herein by reference in its entirety.
[0164] Table 1. Tetrahydrocarbazole acetamide
[0165]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap