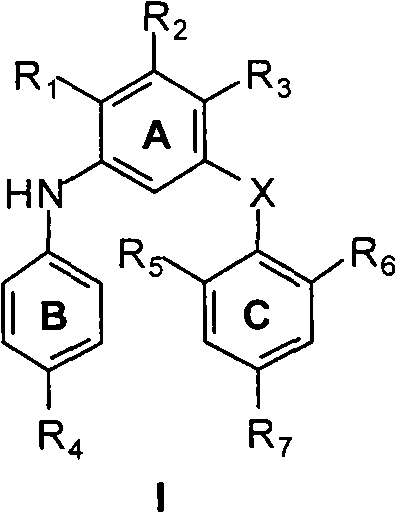
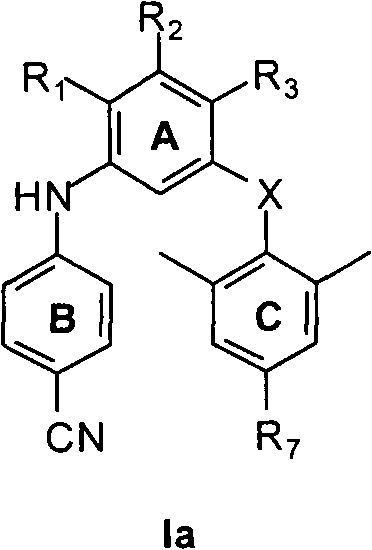
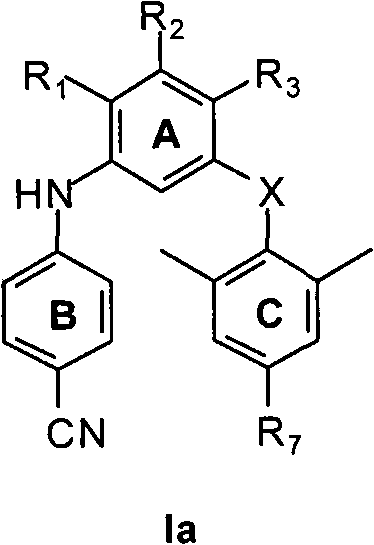
M-bis(arene) polysubstituted amino benzene compound as non-nucleoside HIV reverse transcriptase inhibitor, and preparation and use thereof
一种化合物、烃基的技术,应用在杂环化合物有效成分、有机化学、抗病毒剂等方向,能够解决易产生耐药性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0135] Preparation Example 1: 5-chloro-N-(4'-cyanophenyl)-2,4-dinitroaniline (IV-1)
[0136] 2,4-dichloro-1,5-dinitrobenzene (II-1, 1.2g, 5mmol) and p-cyanoaniline (III-1, 0.59g, 5mmol) were dissolved in N,N-dimethylformaldehyde Amide (DMF, 10 mL). Potassium tert-butoxide (1.4 g, 12.5 mmol) was added in batches while cooling in an ice-water bath, and then reacted at room temperature for 45 minutes. The reaction solution was poured into ice water, the pH value was adjusted to about 6 with dilute HCl, and stirred for 30 minutes to precipitate a solid. The solid was filtered out, washed with water until neutral, dried, and separated on a silica gel column (eluted with ethyl acetate:petroleum ether) to obtain compound VI-1 (1.44 g, 90%) as a pale yellow solid. 1 H NMR (CDCl 3 )δppm, 7.41 (1H, s, ArH-6), 7.57 (2H, d, J=8.7Hz, ArH-3', 5'), 7.91 (2H, d, J=8.7Hz, ArH-2', 6'), 8.90 (1H, s, ArH-3), 10.07 (1H, s, NH).
preparation Embodiment 2
[0137] Preparation Example 2: 5-chloro-2,4-dinitro-N-(4'-methoxyphenyl)-aniline (IV-2)
[0138] 2,4-dichloro-1,5-dinitrobenzene (II-1, 0.5 g, 2.11 mmol) and p-methoxyaniline (III-2, 0.26 g, 2.11 mmol) were dissolved in 5 mL DMSO, Join K 2 CO 3 (0.58g, 4.22mmol) and a catalytic amount of metal Cu were stirred and reacted at 115°C for 2 hours under the protection of nitrogen. Pour into ice water and stir to precipitate a solid. The solid was filtered out, washed with water for several times, dried, and separated on a silica gel column (petroleum ether / ethyl acetate), resulting in a reddish-brown solid (IV-2, 0.68g, 67%). 1 H NMR (CDCl 3 )δppm 3.88 (3H, s, OCH 3 ), 7.02 (1H, s, ArH-6), 7.03 (2H, d, J=8.96Hz, ArH-3', 5'), 7.21 (2H, d, J=8.96Hz, ArH-2', 6 '), 9.07 (1H, s, ArH-3), 9.73 (1H, s, NH).
preparation Embodiment 3
[0139] Preparation Example 3: 5-chloro-2,4-dinitro-N-(4'-methylphenyl)-aniline (IV-3)
[0140] The preparation method is the same as IV-2, and the yield is 70%. 1 H NMRδppm 2.08 (3H, s, CH 3 ), 6.86 (2H, d, J=8.12Hz, ArH-3', 5'), 7.03 (1H, s, ArH-6), 7.10 (2H, d, J=8.12Hz, ArH-2', 6 '), 9.16 (1H, s, ArH-3), 9.71 (1H, s, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com