Heteroarylaminosulfonylphenyl derivatives for use as sodium or calcium channel blockers in the treatment of pain
A heteroaryl, aryl technology, applied in the field of heteroaryl aminosulfonyl phenyl derivatives used as sodium or calcium channel blockers in the treatment of pain, can solve sodium channel blockers and calcium channels Blocker side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
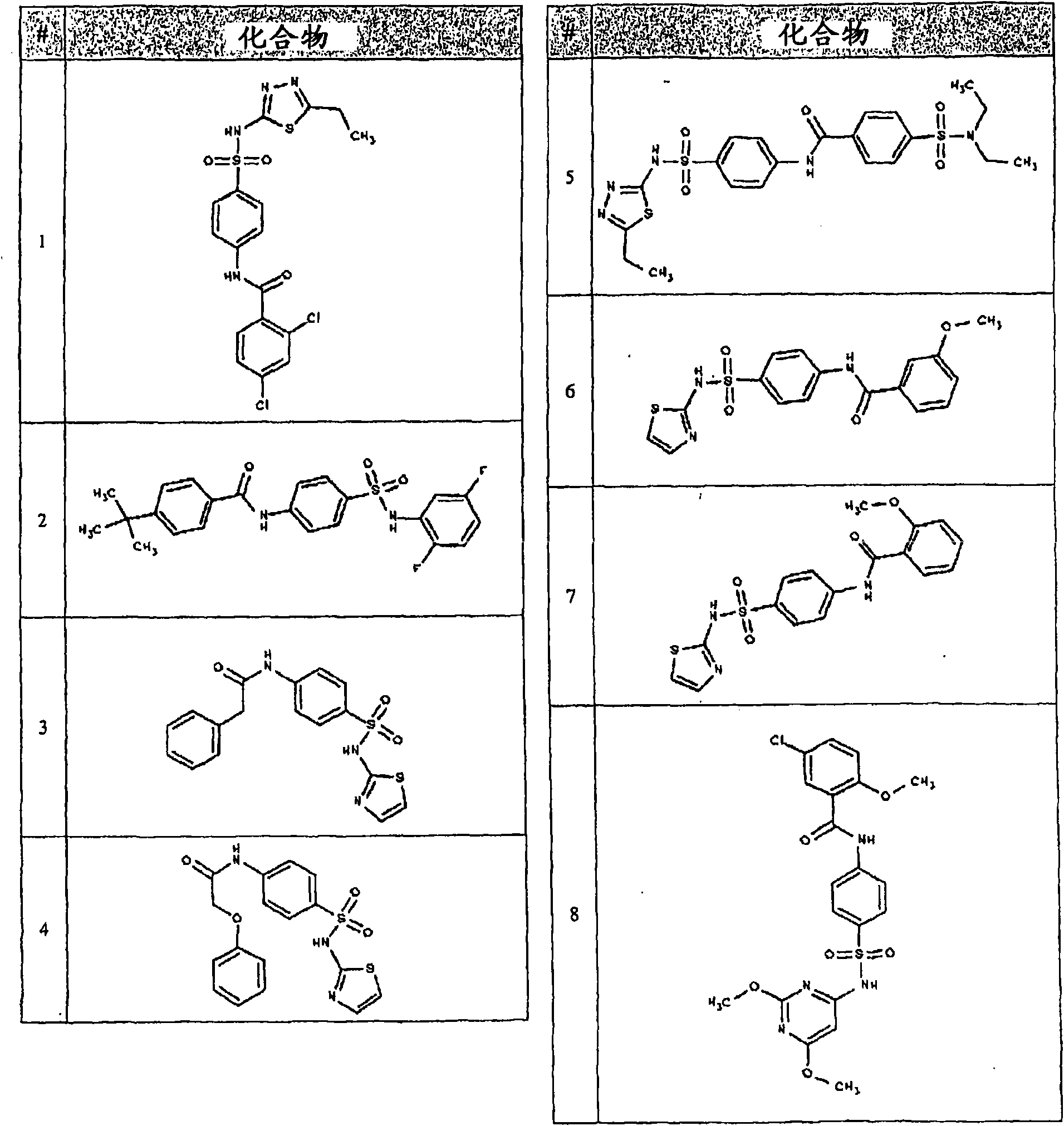
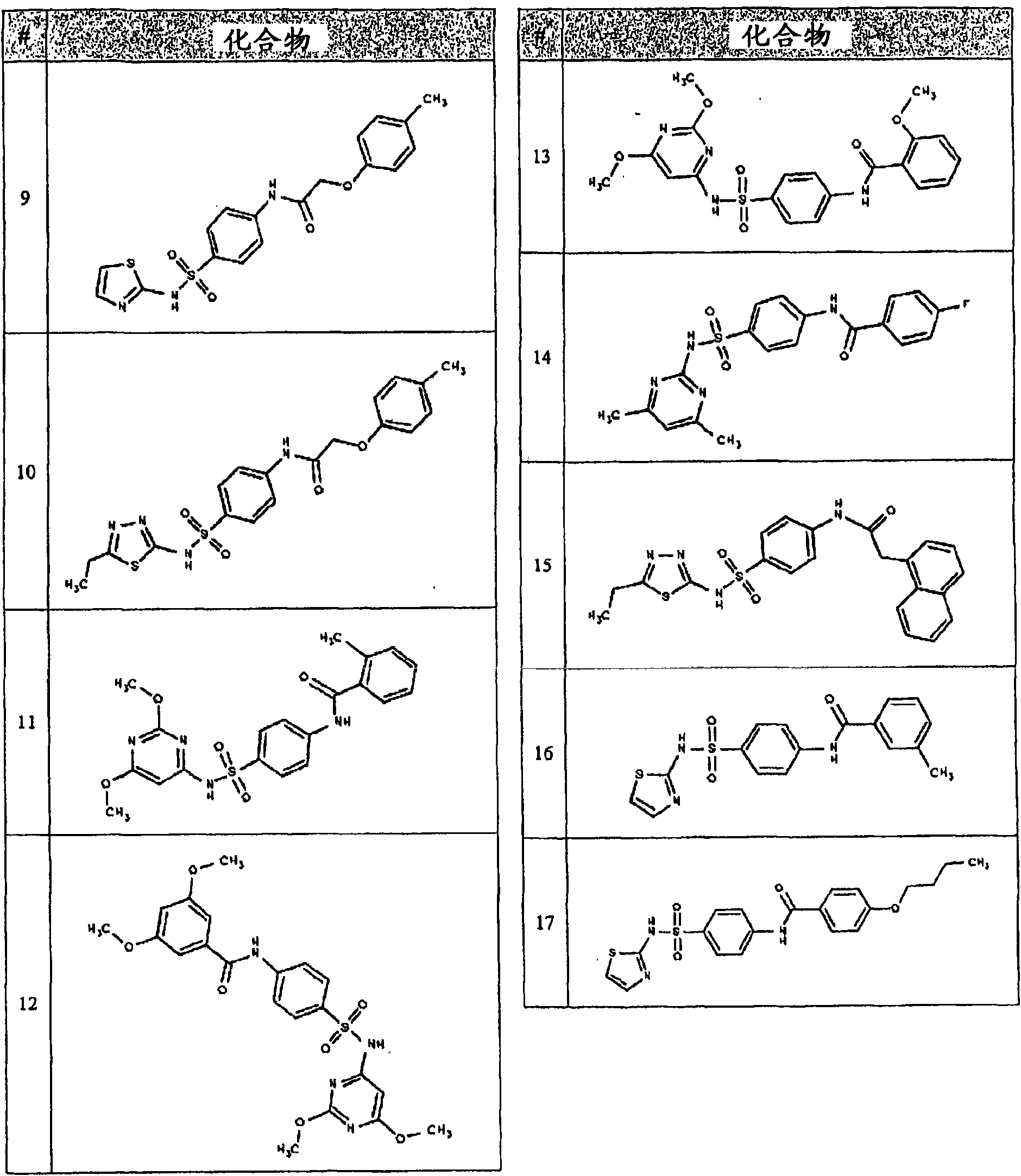
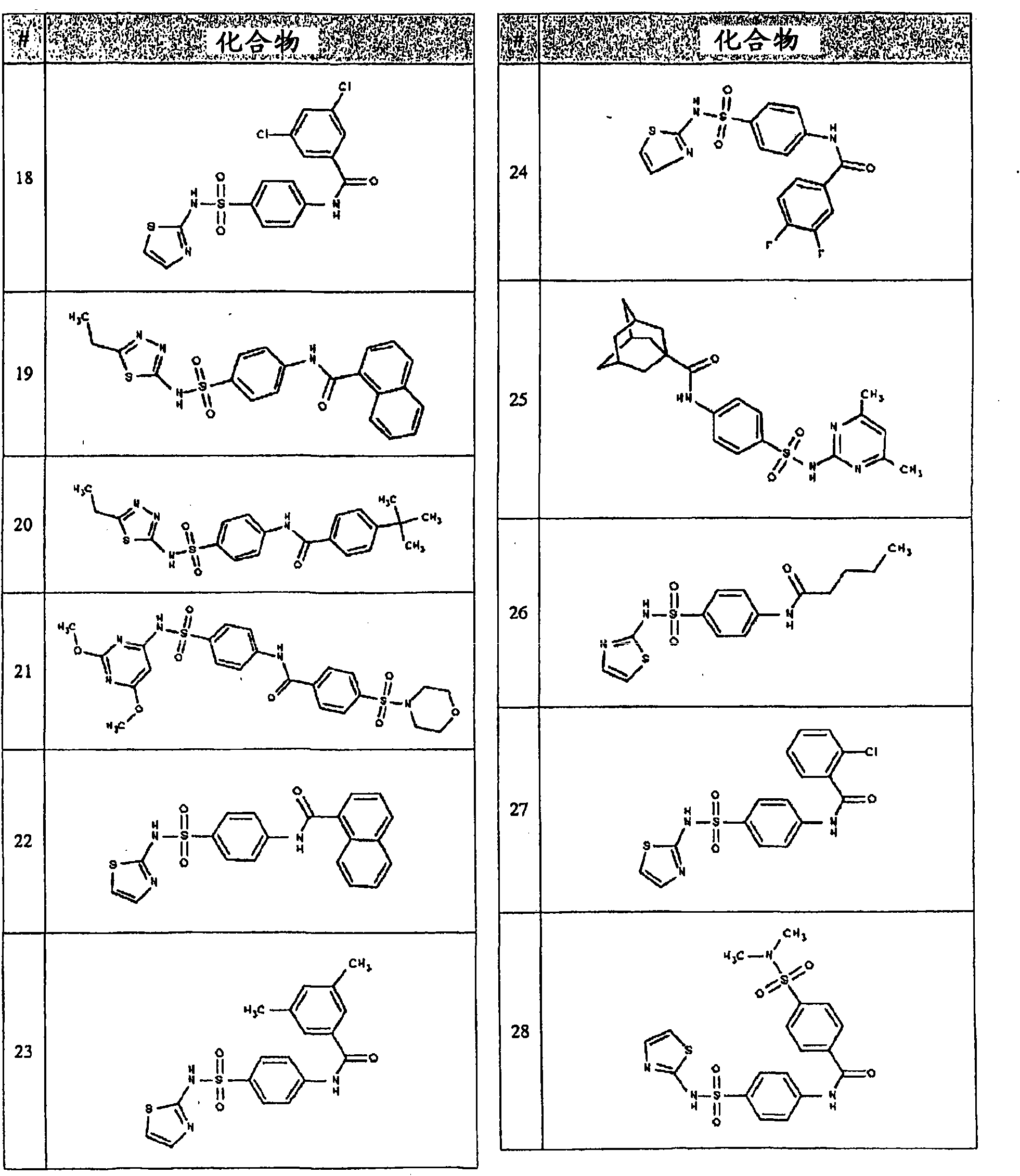
Image
Examples
Embodiment Construction
[0628] 4-(2,4-Dichloro-phenoxy)-butyric acid ethyl ester
[0629]
[0630] At 80°C, to 2,4-dichlorophenol (32.6g, 0.2mol), NaI (3g) and K 2 CO 3 (69 g, 0.5 mol) in DMF (500 mL) was added dropwise with ethyl 4-bromobutyrate (39 g, 0.2 mol). The reaction mixture was stirred at 80 °C for 2 hours until the reaction mixture became colorless. The cooled mixture was filtered and the filtrate was diluted with EtOAc (1000 mL), washed with water (3 x 500 mL), dried and concentrated to give crude butyrate (57 g) as a colorless oil.
[0631] 1 H-NMR (CDCl 3 ): δ7.34(d, 1H, J=8.8Hz), 7.16(dd, 1H, J 1 =8.8Hz,J 2 =2.4Hz), 6.84(d, 1H, J=8.8Hz), 4.15(q, 2H, J=7.2Hz), 4.06(t, 2H, J=7.2Hz), 2.54(t, 2H, J=7.2 Hz), 2.17(p.2H, 6.4), 1.25(t, 3H, J=7.2Hz).
[0632] 4-(2,4-Dichloro-phenoxy)-butanoic acid
[0633]
[0634] Add LiOH.H 2 O (12.6 g, 0.3 mol), the reaction mixture was stirred at room temperature (RT) for 5 hours. The mixture was washed with Et 2 O washed (3 x 200 mL), aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com