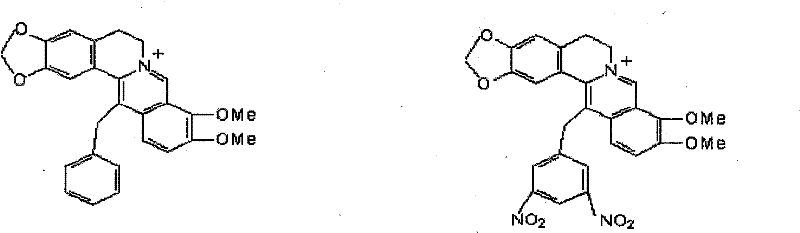
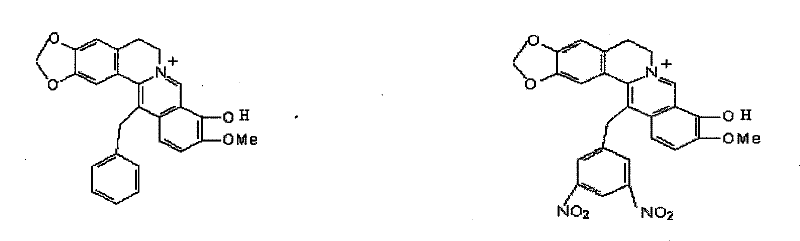
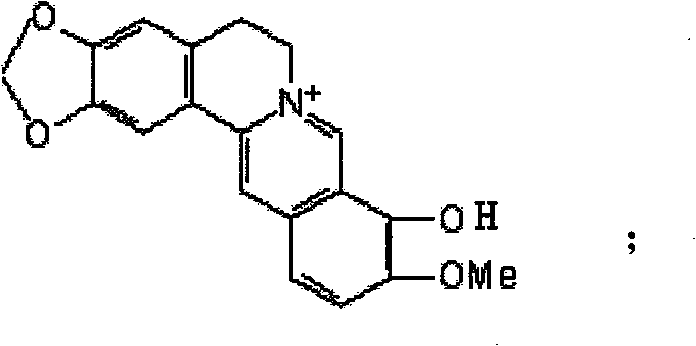
Synthetic method of berberine 13-bit derivant and berberrubine 13-bit derivant
A technology of berberine and synthesis method, which can be applied in the directions of antifungal agents, organic chemistry, antibacterial drugs, etc., and can solve the problems of reducing activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Preparation of 8-acetyl berberine
[0047] Weigh 0.5g of berberine into a 50ml three-neck flask, add 20ml of sodium hydroxide solution with a molar concentration of 1mol / L to dissolve it completely, slowly add 0.11mL of acetone dropwise at a rate of 0.17ml / min, and place at 24°C The reaction was stirred for 4 hours to obtain 0.25 g of 8-acetyl berberine. The yield was 42%.
Embodiment 2
[0048] Embodiment 2: Preparation of 8-acetyl berberine
[0049] Weigh 0.5g of berberine into a 50mL three-neck bottle, add 20mL of sodium hydroxide solution with a molar concentration of 4mol / L to dissolve it completely, slowly add 0.33mL of acetone dropwise at a rate of 0.17ml / min, and store at 24°C The reaction was stirred for 4 hours to obtain 0.49 g of 8-acetyl berberine. The yield was 84%.
Embodiment 3
[0050] Embodiment 3: the preparation of 8-acetyl berberine
[0051] Weigh 0.5g of berberine into a 50mL three-necked bottle, add 20mL of sodium hydroxide solution with a molar concentration of 7mol / L to dissolve it completely, slowly add 0.55ml of acetone dropwise at a rate of 0.17ml / min, and stir at 24°C After reacting for 4 hours, 0.39 g of 8-acetyl berberine was obtained. The yield is 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com