Methods of modifying antibodies, and modified antibodies with improved functional properties
一种氨基酸、编号系统的技术,应用在修饰抗体和具有改善的功能性质的修饰抗体领域,能够解决蛋白质弱稳定性和溶解性、高聚集、增加免疫原性危险等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0567] It is to be understood that the present invention also includes any of the methods, references and / or compositions shown in Appendices (A-C) of U.S. Provisional Patent Application Serial No. 60 / 905,365 and Appendices (A-I) of U.S. Provisional Patent Application Serial No. 60 / 937,112 , including but not limited to, identified databases, bioinformatics, in silico data manipulation and interpretation methods, functional assays, preferred sequences, preferred residue positions / changes, framework identification and selection, framework changes, CDR alignment and integration and preferred changes / mutations.
[0568]Additional information on such methods and compositions can be found in U.S.S.N.s 60 / 819,378; and U.S.S.N.s entitled "scFv Antibodies Which Pass Epithelial And / Or Endothelial Layers," filed July 2006 and February 6, 2007, respectively. 60 / 899,907 and PCT Publication No. WO 2008 / 006235; WO06131013A2, filed June 6, 2006, entitled "Stable And Soluble Antibodies Inhibi...
Embodiment 1
[0572] Example 1: Antibody position numbering system
[0573] In this example, a conversion table for two different numbering systems used to identify amino acid residue positions in antibody heavy and light chain variable regions is provided. The Kabat numbering system is further described in Kabat et al. (Kabat, E.A., et al. (1991) Sequences of Proteins of Immunological Interest, 5th Edition, U.S. Department of Health and Human Services, NIH Publication No. 91-3242). The AHo numbering system is further described by Honegger, A. and Plückthun, A. (2001) J. Mol. Biol. 309 : 657-670).
[0574] Heavy chain variable region number
[0575] Table 1: Conversion table for residue positions in the heavy chain variable domain
[0576] Kabat AHo
Kabat AHo
Kabat AHo
1 1
twenty two
3 3
4 4
5 5
6 6
7 7
· 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 1...
Embodiment 2
[0586] Example 2 : Sequence-based analysis of scFv sequences
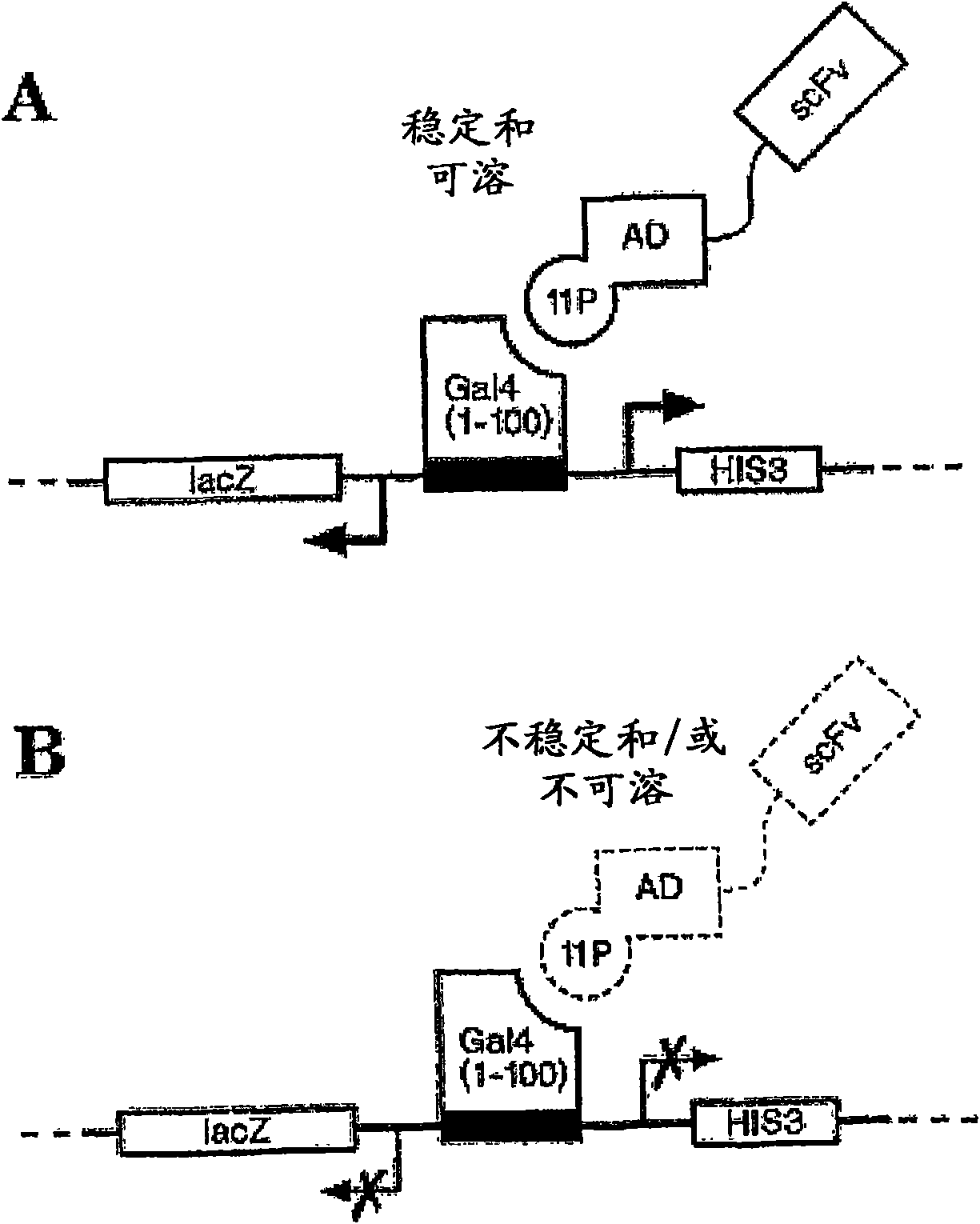
[0587] In this example, sequence-based analysis of scFv sequences is described in detail. A flowchart outlining the analysis process is shown in figure 1 middle.
[0588] Collection and Alignment of Human Immunoglobulin Sequences
[0589] Human mature antibody and germline variable domain sequences were collected from various databases and entered into custom databases as single letter coded amino acid sequences. Antibody sequences were aligned using the EXCEL tool of the Needleman-Wunsch sequence alignment algorithm (Needleman et al., J Mol Biol., 48(3):443-53 (1970)). The database is then subdivided into 4 different arrays (according to the original data source) as follows to facilitate subsequent analysis and comparison:
[0590] VBase : human germline sequence
[0591] IMGT : human germline sequence
[0592] KDB Database: Mature Antibodies
[0593] QC Database: ESBATech internal database con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com