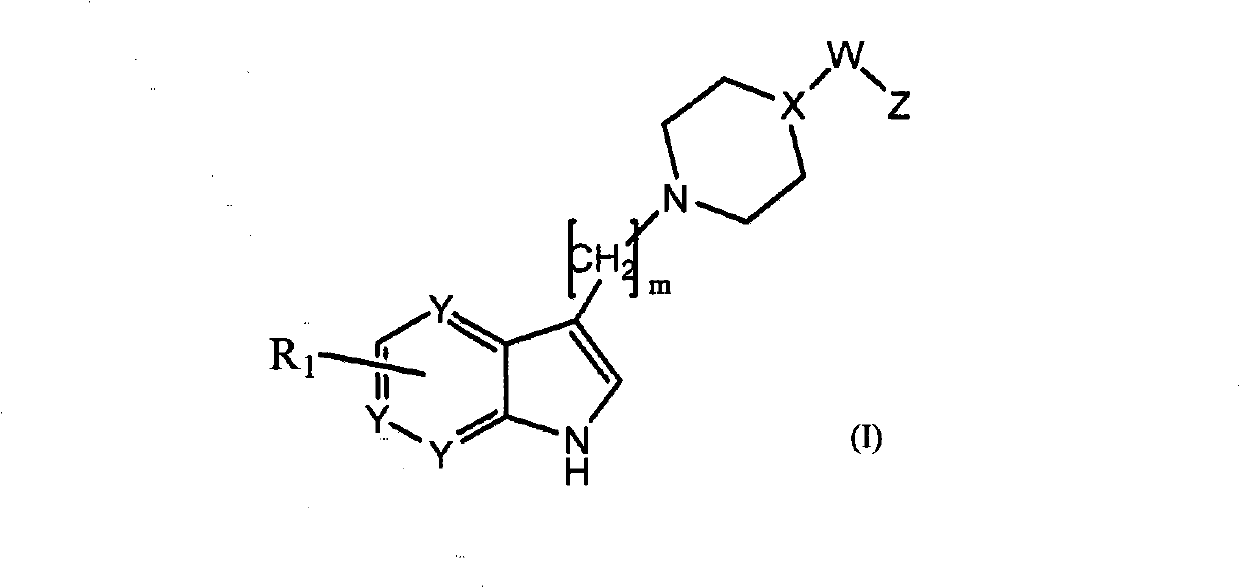
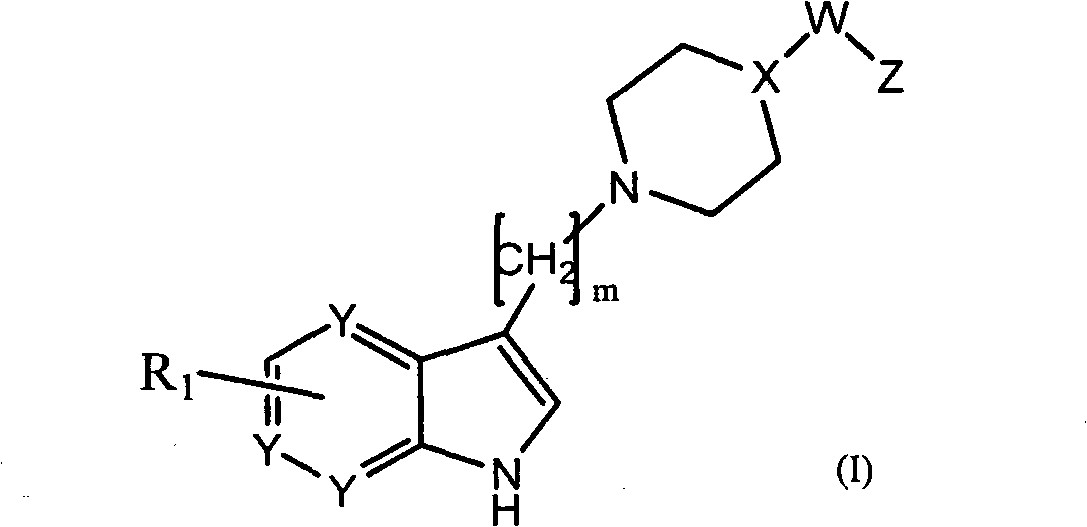
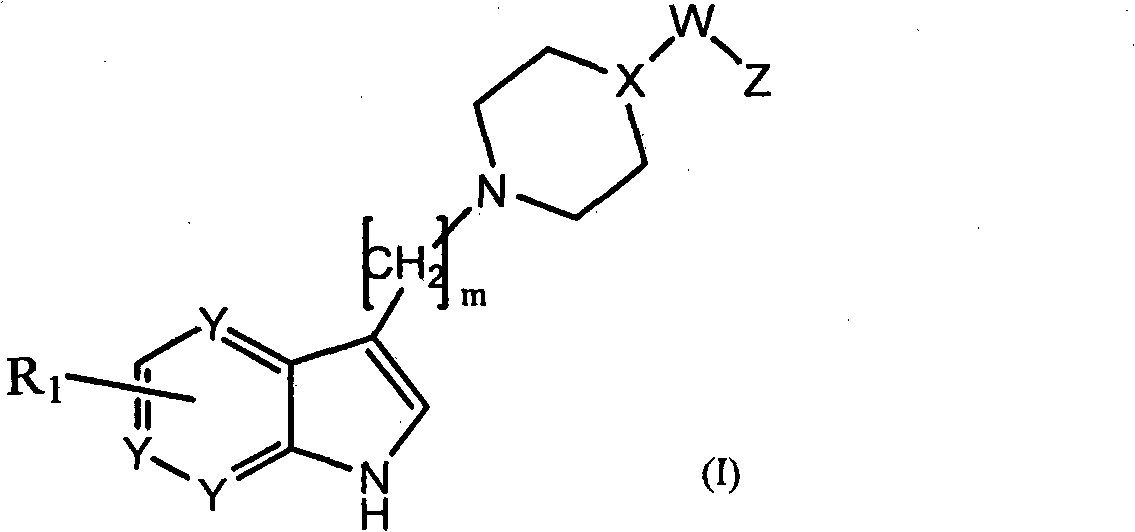
Azaindole compounds for treatment of central nervous system disorders
A technology of compounds and mixtures, applied in the field of pharmaceutical compounds for the treatment of central nervous system dysfunction, can solve problems such as slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] 5-{4-[4-(5-cyano-1H-pyrrolo[2,3-b]pyridin-3-yl)-butyl]-piperazin-1-yl}-benzofuran-2- Synthesis of formamide:
[0131]
[0132] a.: Dissolve 67 g of 6-amino-nicotine nitrile in 1 L of 1,2-dichloroethane, add 125 g of silver trifluoroacetate, and reflux the mixture for 7 hours. After cooling to room temperature (RT), 143 g of iodine were added. The mixture was heated again for 12 hours. The temperature was then lowered to RT and the salts were removed by filtration. The reaction phase was treated with 1 L of water. The aqueous phase was extracted with dichloromethane, the combined organic layers were dried over magnesium sulfate, evaporated and purified by chromatography on silica gel to give 41 g of 6-amino-5-iodo-nicotinenitrile as pale yellow crystals.
[0133] [M+H] + :246
[0134] b.: 6 g of 6-chloro-1-hexyne were dissolved in 50 ml THF and cooled to -78°C. 31 ml of n-BuLi (1.6M in hexane) was added dropwise at this temperature. The reaction mixture was he...
Embodiment 2
[0154] 5-{4-[4-(5-cyano-1H-pyrrolo[3,2-b]pyridin-3-yl)-butyl]-piperazin-1-yl}-benzofuran-2- Synthesis of formamide:
[0155]
[0156] 25 g of 5-amino-pyridine-2-carbonitrile were treated as described for 6-amino-nicotine nitrile to afford 20 g of 5-amino-6-iodo-pyridine-2-carbonitrile as light tan crystals.
[0157] HPLC: Chromolite Performance RP18-e 100-4, 6mm
[0158] Gradient: ACN / 0.05% formic acid in water
[0159] Method: Chromolith / Chromolith (Extended)
[0160] Flow rate: 3mL / min
[0161] Retention value (Rt): 1.596min
[0162] [M+H] + :246
[0163] 1 H-NMR (500MHz, d 6 -DMSO) δ 7.62 (d, 1H, J=8.3Hz), 6.97 (d, 1H, J=8.3Hz), 6.44 (br.s, 2H).
[0164] According to 5-{4-[4-(5-cyano-1H-pyrrolo[2,3-b]pyridin-3-yl)-butyl]-piperazin-1-yl}-benzofuran- 500 mg of 5-amino-6-iodo-pyridine-2-carbonitrile and 1.2 g of 5-[4-(6-trimethylsilyl-hexyn-5-yl)-piperamide were treated as described in 2-carboxamide Azin-1-yl]-benzofuran-2-carboxamide to give 20 mg of 5-{4-[4-(5-cy...
Embodiment 3
[0175] 3-{4-[4-(2,3-dihydro-benzo[1,4]dioxane-6-yloxy)-piperidin-1 base]-butyl}-1H-pyrrole[ Synthesis of 2,3-b]pyridine-5-carbonitrile
[0176]
[0177] 10 g of 5-hexyn-1-ol was dissolved in 150 mL THF and cooled to -78°C. 187 ml (1.6M in n-hexane) of butyllithium were added dropwise. After stirring at -20°C for 1 hour, 30 ml of trimethylchlorosilane was added dropwise at the given temperature. After 12 h at RT, the mixture was worked up with 100 mL of water. After the usual extraction and purification procedure 3.3 g of 6-trimethylsilyl-hex-5-yn-1-ol were obtained as a colorless oil.
[0178] According to 5-{4-[4-(5-cyano-1H-pyrrolo[2,3-b]pyridin-3-yl)-butyl]-piperazin-1-yl}-benzofuran- 2.9 g of 6-amino-5-iodo-nicotinenitrile and 2 g of 6-trimethylsilyl-hex-5-yn-1-ol were treated with 2-carboxamide as described to give 630 mg of 3-( 4-Hydroxy-butyl)-2-trimethylsilyl-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile.
[0179] HPLC: Rt: 2.370min
[0180] HPLC-MS: Rt: 1.429min ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap