Monoazo and polyazo dyes favorable for health and synthesis method thereof
A synthesis method and polyazo technology, applied in monoazo dyes, azo dyes, organic dyes, etc., can solve problems such as carcinogenesis, and achieve the effect of ensuring beneficial effects and improving living standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0039] The present invention will be further described below in conjunction with embodiment:
[0040] 1. Some aminochalcone derivative intermediates carry out the synthesis of mono- and polyazo healthy dye structures:
Embodiment 1
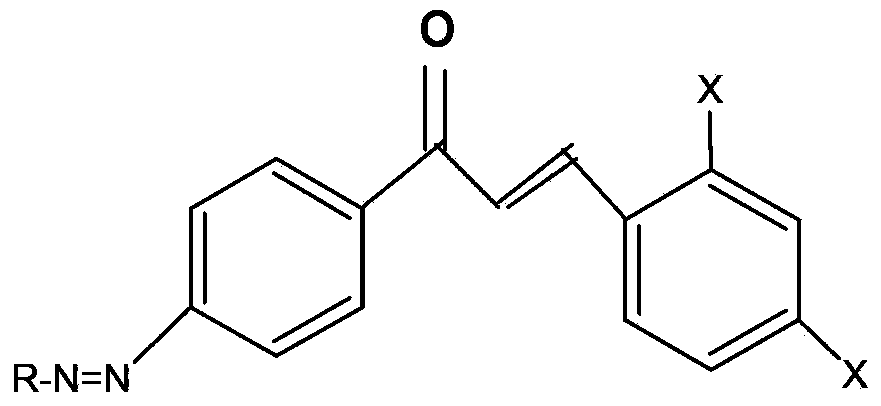
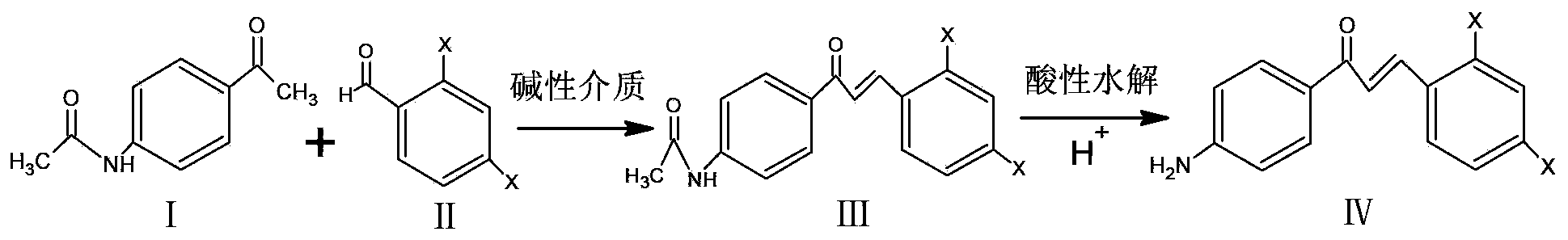
[0042] (1) Synthesis of dye intermediates containing amino-containing chalcone (such as: 2,4-dichloro-4'-aminochalcone) dye intermediates, the reaction formula is:
[0043]
[0044] Operation steps: Take p-acetamidoacetophenone, methanol and 0.5mol / L sodium hydroxide solution and stir at room temperature to form a homogeneous phase, then add the mixture of 2,4-dichlorobenzaldehyde and methanol dropwise under stirring in an ice bath, and stir for 15 hours, poured into water, adjusted to weak alkaline with acid, filtered with suction, washed with water until neutral, and recrystallized with ethanol to obtain light yellow crystal 2,4-dichloro-4'acetamidochalcone.
[0045] Take 2,4-dichloro-4'acetamidochalcone and ethanol, heat to boiling, add acid dropwise, stir under reflux for 8 hours, cool naturally, pour into water, adjust to neutral, suction filter, dry, Recrystallization with methanol gave orange crystals, 2,4-dichloro-4'-aminochalcone intermediate.
[0046] (2) Amino-c...
Embodiment 2
[0053] Embodiment 2: Using intermediate I as a raw material and pyrazolone as a coupling component, a yellow healthy dye is synthesized;
[0054] (1) the synthesis of the chalcone dye intermediate containing amino, with embodiment 1,
[0055] (2) Intermediate I is made into diazonium salt, same as Example 1;
[0056] (3) Synthetic yellow dye with pyrazolone as the coupling component: the reaction formula is:
[0057]
[0058] Operation steps: Add a certain amount of 80°C hot water, Na 2 CO 3 Solid, 2.0g pyrazolone, stir to dissolve, cool down to 10°C, and evenly add the prepared diazonium salt solution into the three-necked flask in about 50 minutes, keep the temperature at 5°C~10°C during the addition process, And make the pH=8.0~9.0, stir the reaction to the end of the reaction. After the end point is reached, the pH of the reaction solution is adjusted to 7, the temperature is raised to 80° C., and the salt content of 20% of the volume of the solution is controlled f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com