Application of astragaloside IV in preparing liver cancer resisting medicines
A technology for astragalus saponins and medicines is applied in the application field of astragalus saponins IV in the preparation of anti-cancer drugs, and can solve the problems of control, difficult quality and stability, complex components and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
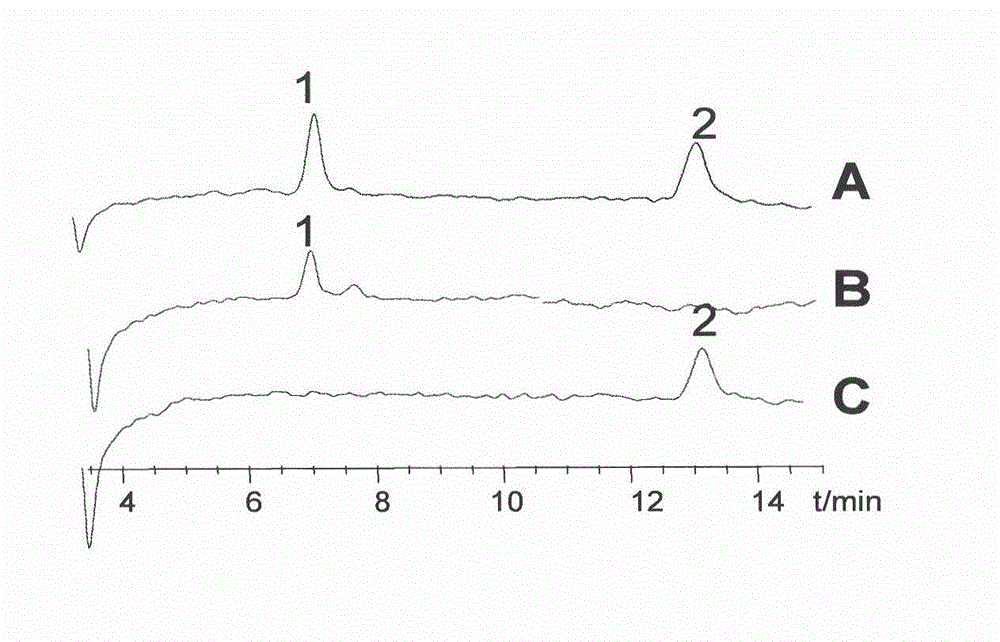
[0054] Example 1 Preparation of Astragaloside II and IV
[0055] 1. Experimental materials
[0056] 1.1 Test drug Astragalus (origin Gansu, purchased from Guotou Pharmaceutical Anhui Co., Ltd., batch number: 080623; identified as Astragalus membranaceus by Professor Liu Shoujin, Department of Medicinal Botany, Anhui College of Traditional Chinese Medicine); Astragaloside IV standard product (China Pharmaceutical Biology Product Inspection Institute, batch number: 0781-200210); astragaloside Ⅱ standard product (provided by Shanghai Standardization Research Center of Traditional Chinese Medicine, batch number: 07-1015); high-efficiency silica gel G plate (Qingdao Ocean Chemical Factory); silica gel for column chromatography (Qingdao Ocean Chemical Group); D101 resin (Tianjin Youchang Industry and Trade Co., Ltd.); ethanol, n-butanol, ethyl acetate, and methanol are analytically pure, acetonitrile is chromatographically pure, and water is double distilled water.
[0057] 1.2 Apparatus ...
Embodiment 2
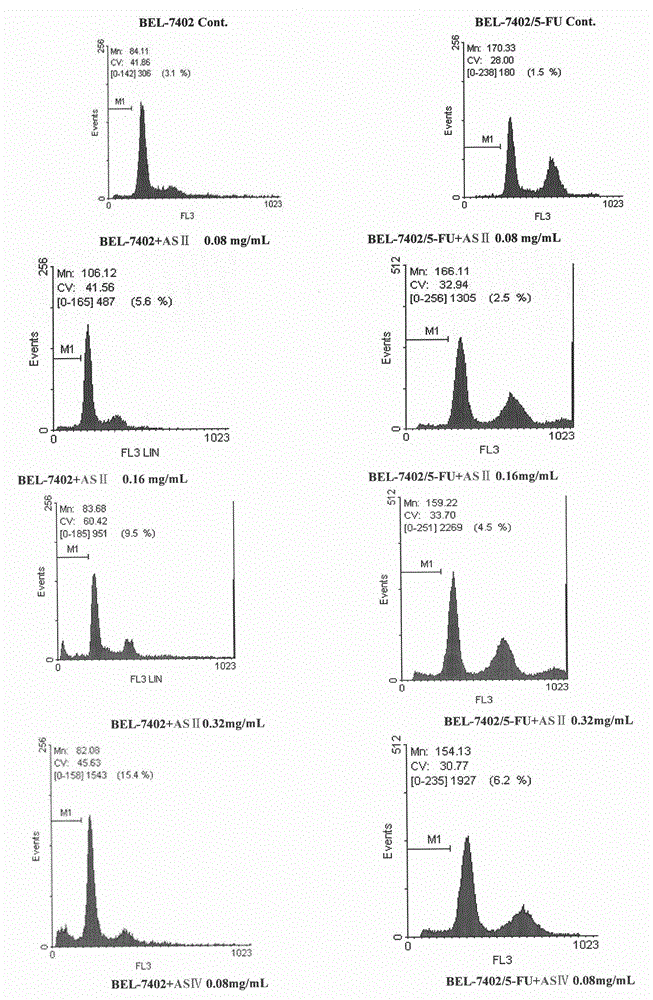
[0103] Example 2 Study on the anti-tumor effect of astragaloside Ⅱ and Ⅳ
[0104] 1. Experimental materials
[0105] 1.1. Cell lines Human liver cancer cell line BEL-7402 and liver cancer drug-resistant cell line BEL-7402 / 5-FU were purchased from Nanjing KGI Biotechnology Development Co., Ltd.
[0106] 1.2 Medicines and reagents
[0107] Astragaloside Ⅱ (AS Ⅱ), Astragaloside Ⅳ (AS Ⅳ) (laboratory self-made)
[0108] Right-handed verapamil (R(+)-VERAPAMIL HCL, VRP) was purchased from IL company in the United States, purity>99%
[0109] 5-FU Tianjin Jinyao Amino Acid Co., Ltd., batch number 0810211
[0110] MMC Zhejiang Hisun Pharmaceutical Co., Ltd., batch number 0810211
[0111] ADR Zhejiang Hisun Pharmaceutical Co., Ltd., batch number 0810211
[0112] DMEM and Fetal Bovine Serum American Gibco products
[0113] MTT American Sigma company product
[0114] DMSO American Sigma company product
[0115] Rho123 American Sigma company product
[0116] Trypsin BIOSHARP company product
[0117] PBS Boste...
Embodiment 3
[0249] Tablet: Astragaloside IV 10mg
[0250] Lactose 187mg
[0251] Corn starch 50mg
[0252] Magnesium stearate 3mg
[0253] Preparation method: mix the active ingredient, lactose and starch, moisten it evenly with water, sieve and dry the moistened mixture, sieve again, add magnesium stearate, then press the mixture into tablets, each tablet weighs 250 mg, and the active ingredient content It is 10mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com