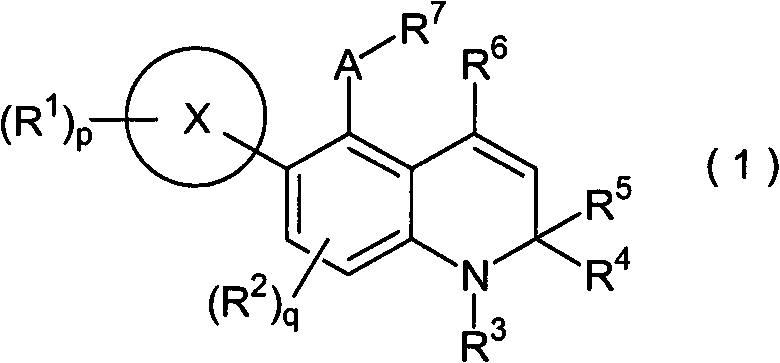
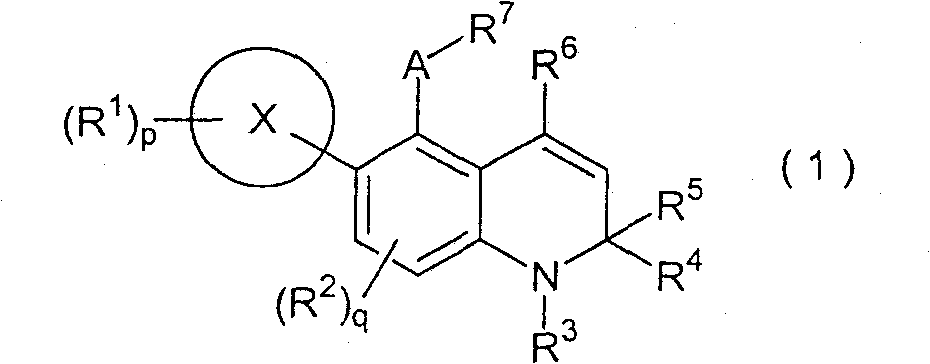
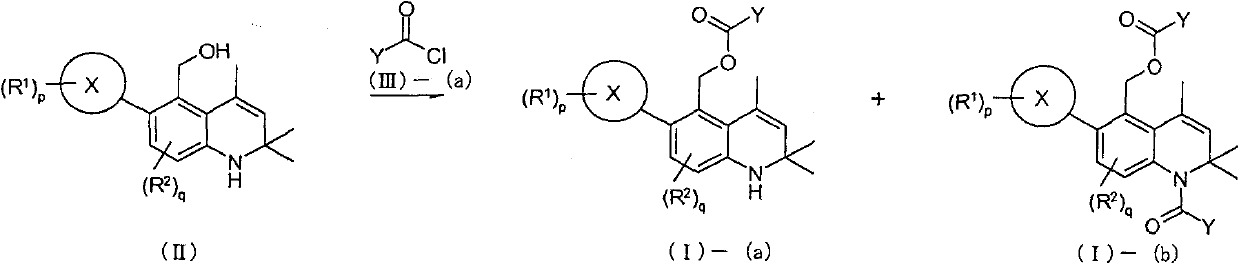
Novel 1-2-dihydroquinoline derivative having glucocorticoid receptor binding activity
A compound, C1-C8 technology, applied in drug combinations, skin diseases, organic chemistry, etc., to achieve excellent glucocorticoid receptor binding activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0353] 2,2,4-Trimethyl-1,2-dihydro-6-oxa-1-aza -5-one (reference compound 1-1)
[0354] Methyl 2-(2-benzyloxyphenyl)-5-nitrobenzoate (reference compound 1-1-(1))
[0355] 2-benzyloxyphenylboronic acid (20.2g, 88.6mmol), methyl 2-bromo-5-nitrobenzoate (25.4g, 97.5mmol), cesium carbonate (57.7g, 177mmol) and bis(triphenyl A mixture of phosphine)palladium(II) dichloride (1.16g, 1.65mmol) was suspended in anhydrous N,N-dimethylformamide (300mL), and stirred at 80°C for 3 days under an argon atmosphere. After standing to cool, ethyl acetate (500 mL), diethyl ether (300 mL) and water (500 mL) were added and partitioned. The aqueous layer was extracted with a mixture of ethyl acetate (200 mL) and diethyl ether (200 mL), and the organic layers were combined. The organic layer was washed successively with water (500 mL, twice) and saturated brine (300 mL), dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was pu...
reference example 2
[0426] 7-methoxy-2,2,4-trimethyl-1,2-dihydro-6-oxa-1-aza -5-ketone (reference compound 2-1)
[0427] 7-Hydroxy-2,2,4-trimethyl-1,2-dihydro-6-oxa-1-aza A mixture of -5-ketone (reference compound 1-9, 430mg, 1.40mmol), iodomethane (87.2μl, 1.40mmol) and potassium carbonate (387mg, 2.80mmol) was suspended in anhydrous N,N-dimethylformamide (7 mL), stirred at 50°C for 3 hours. After cooling, ethyl acetate (150 mL) was added for dilution. After successively washing with water (150 mL) and saturated brine (50 mL), and drying over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure. The resulting residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain the title reference compound (384 mg) as a yellow solid. (yield 85%)
[0428]
[0429] Hereinafter, reference compounds 2-2 to 2-6 were obtained according to the production method of reference compound 2-1 using a compound selected from reference compounds 1-9, 1-...
reference example 3
[0433] 5-Hydroxymethyl-6-(2-hydroxyphenyl)-2,2,4-trimethyl-1,2-dihydroquinoline (reference compound 3-1)
[0434] Under an argon atmosphere, lithium aluminum hydride (1.48 g, 39.0 mmol) was suspended in anhydrous tetrahydrofuran (30 mL). Add 2,2,4-trimethyl-1,2-dihydro-6-oxa-1-aza dropwise at 0°C - A solution of 5-ketone (reference compound 1-1, 3.80 g, 13.0 mmol) in anhydrous tetrahydrofuran (40 mL) was stirred at the same temperature for 1 hour. Ethyl acetate (15 mL) and water (5 mL) were successively added dropwise to the reaction solution, and then 0.2N hydrochloric acid (350 mL) was added. After extraction with ethyl acetate (300 mL, 100 mL), the organic layers were combined. The organic layer was washed successively with water (300 mL) and saturated brine (100 mL), and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain the title reference compound (4.01 g) as a light brown solid. (quantitative)
[0435]
[0436...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap