Method of treating asthma with antiviral agents
An antiviral agent, a technology for asthma, applied in the field of asthma treatment, can solve problems such as unsuitability for maintenance treatment and pulmonary function decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] An adult male with a history of asthma has been taking inhaled steroids and beta 2 - Agonist for many years, with mixed results. he used to use inhaled twice daily, or Spray four times a day. is a drug containing fluticasone propionate (a corticosteroid) and salmeterol xinafoate (a long-acting beta 2 - combination preparations of adrenergic receptor agonists). Contains two active ingredients delivered through a single inhaler: budesonide - a corticosteroid, and formoterol - a long-acting beta 2 - Agonists. His peak flow is at best 600-650 L / min, but will degenerate to around 250-350 L / min during exacerbations, and usually drops below 550 L / min when not having seizures. Over the years, he required occasional treatment with oral prednisone, a corticosteroid. Exercise tolerance was moderate to poor.
[0094] He exhibited flu-like symptoms and was started on Tamiflu 75mg, twice a day for 5 days. Within 12 hours, he began to feel better and his myalgia decreas...
Embodiment 2
[0095] Embodiment 2 oseltamivir phosphate (tamiflu ) randomized, double-blind, placebo-controlled evaluation in patients with poorly controlled asthma
[0096] Current asthma guidelines emphasize asthma control. One aspect of control is the current burden indicated by symptom levels, rescue medication use, nighttime awakenings, and interference with normal activities. Patients who did not meet the specified goals were considered to have "not well controlled asthma". The guidelines further recommend treatment steps to achieve control. For many patients, these measures are sufficient to achieve asthma control, but for at least 30% of patients, even optimal doses of inhaled corticosteroids and long-acting beta-agonists sustained for one year were not achieved in the GOAL study Control (Bateman et al., Can guideline defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med2004;170:836-44). Therefore, there is a need to identify ...
Embodiment 3
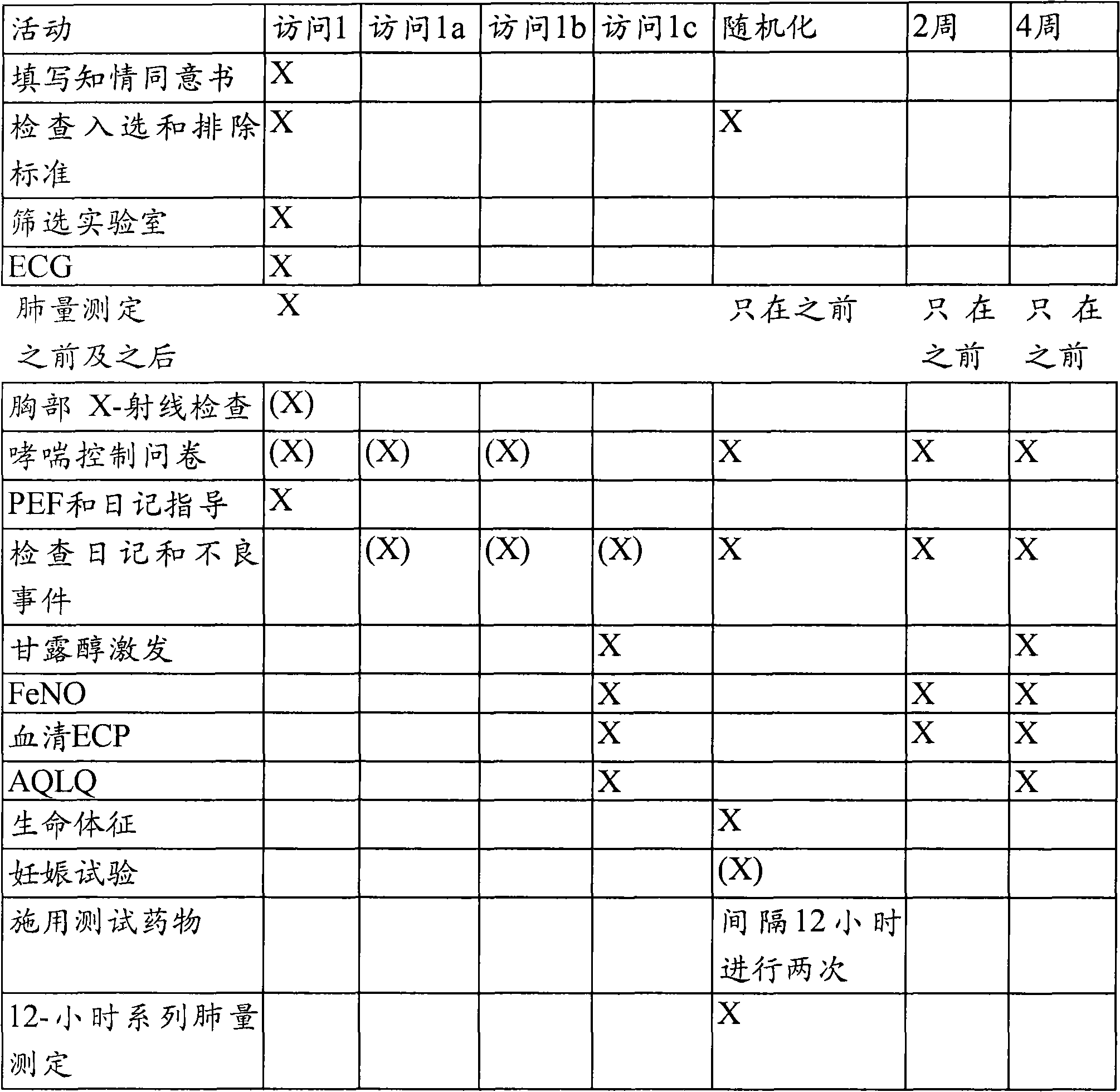
[0165] Twelve patients with asthma were given an appropriate dose, e.g., the Prescribing Information (PI) dose of oseltamivir phosphate (Tamiflu )treat. Patients can be randomized into several groups: the high-dose group will receive oseltamivir 150 mg twice daily or an equivalent dose adjusted for age, weight, and renal function for 5-10 days; the medium-dose group will receive oseltamivir twice daily 110 mg of oseltamivir or an equivalent dose adjusted according to age, weight and renal function for 5-10 days; the standard dose group will receive 75 mg of oseltamivir twice a day or an equivalent dose adjusted according to age, weight and renal function for 5-10 days sky. http: / / clinicaltrials.gov / ct2 / show / NCT00298233.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com