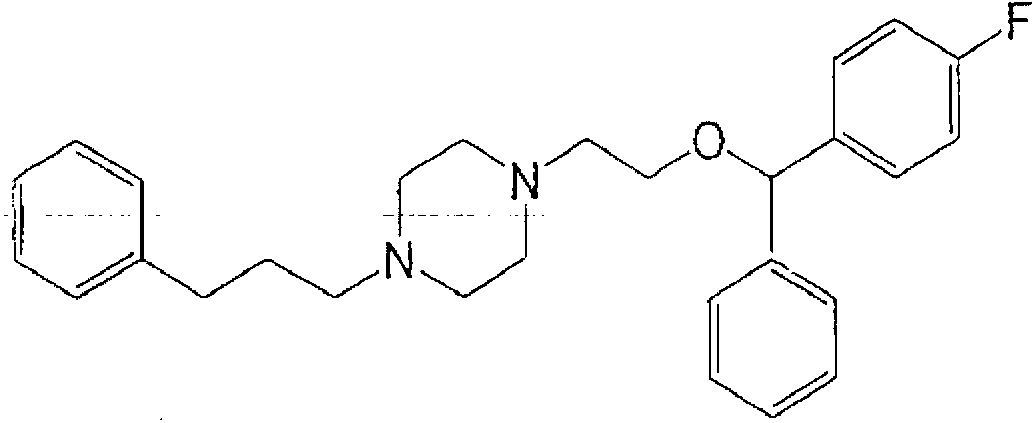
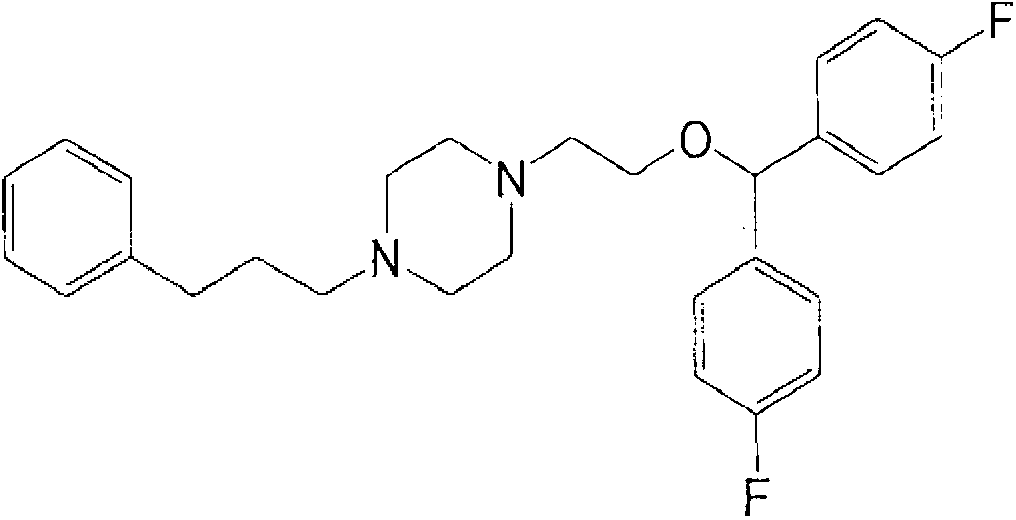
Pharmaceutical compositions for terminating acute episodes of cardiac arrhythmia, restoring sinus rhythm, preventing recurrence of cardiac arrhythmia and/or maintaining normal sinus rhythm in mammals
A technology of mammals and compositions, applied in the direction of drug combinations, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of increasing arrhythmia and losing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] As used herein, the singular forms "a," "an," and "the" include plural references unless the context clearly dictates otherwise.
[0025] As used herein, the term "pharmaceutically acceptable" relates to those compounds, materials, compositions, and / or dosage forms, which are suitable, within the scope of sound medical judgment, for contact with human and animal tissues and / or for use in for human and animal consumption without undue toxicity, irritation, allergic reaction, or other problematic complications commensurate with a reasonable benefit / risk ratio.
[0026] As used herein, "therapeutically effective amount" refers to an amount effective to reduce, eliminate, treat, prevent or manage the symptoms of the diseases and disorders described herein. The term "control" is intended to refer to all processes by which it is possible to slow, interrupt, arrest or stop the progression of the diseases and disorders described herein, but does not necessarily imply complete e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com