Furostan-type saponin derivatives and uses thereof
A furostane type and derivative technology, applied in the field of steroidal saponin derivatives, can solve problems such as no depression, little relationship between chemical structure and pharmacodynamic mechanism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The preparation of embodiment 1 furostane type saponin monosaccharide derivative
[0085]
[0086] Using timosaponin B-Ⅲ as the reaction starting material to prepare furostane-type saponin monosaccharide derivatives, the specific steps are as follows:
[0087] (1) Dissolve 0.05mol timosaponin B-Ⅲ in 300mL methanol solution (water or 30v / v% ethanol solution or 1w / v% Tween solution) with a volume ratio of 10v / v%, and add 0.5mL-1mL Concentrated sulfuric acid, react at 80°C-150°C for 1 hour-6 hours.
[0088] (2) Adjust the above reaction solution to pH 6-7 with sodium hydroxide.
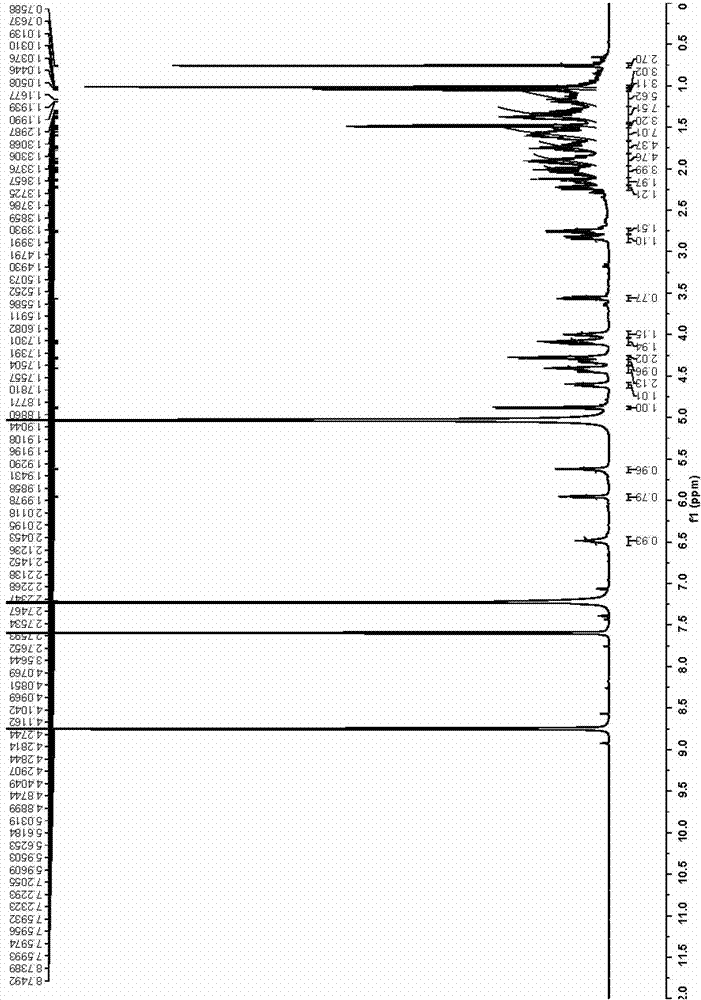
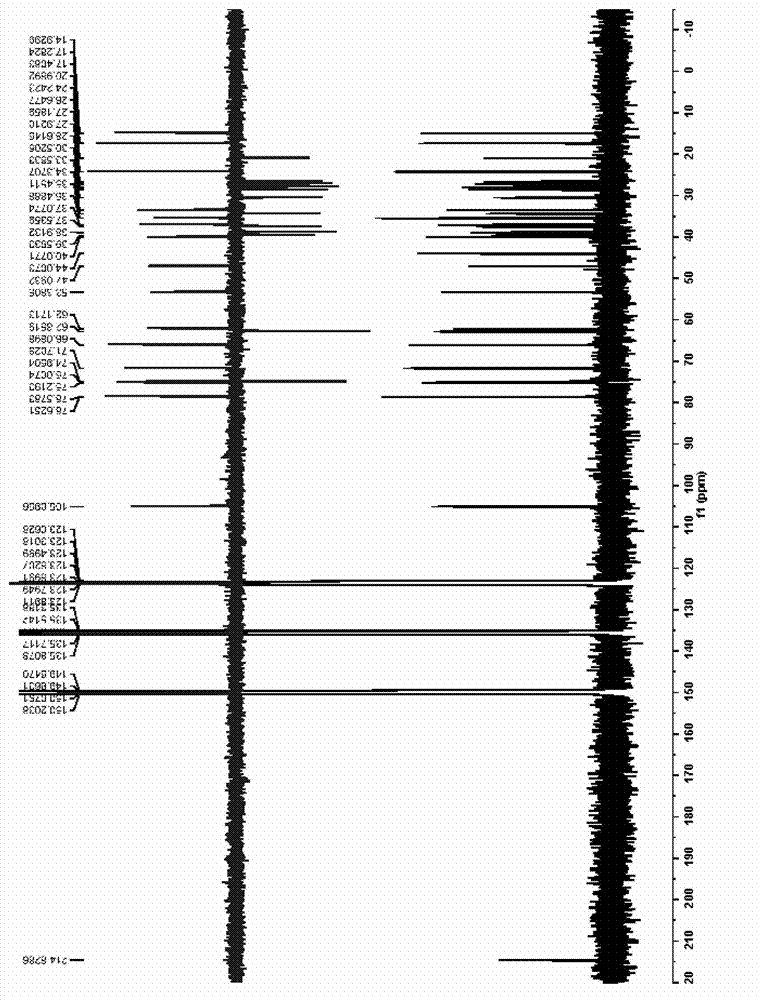
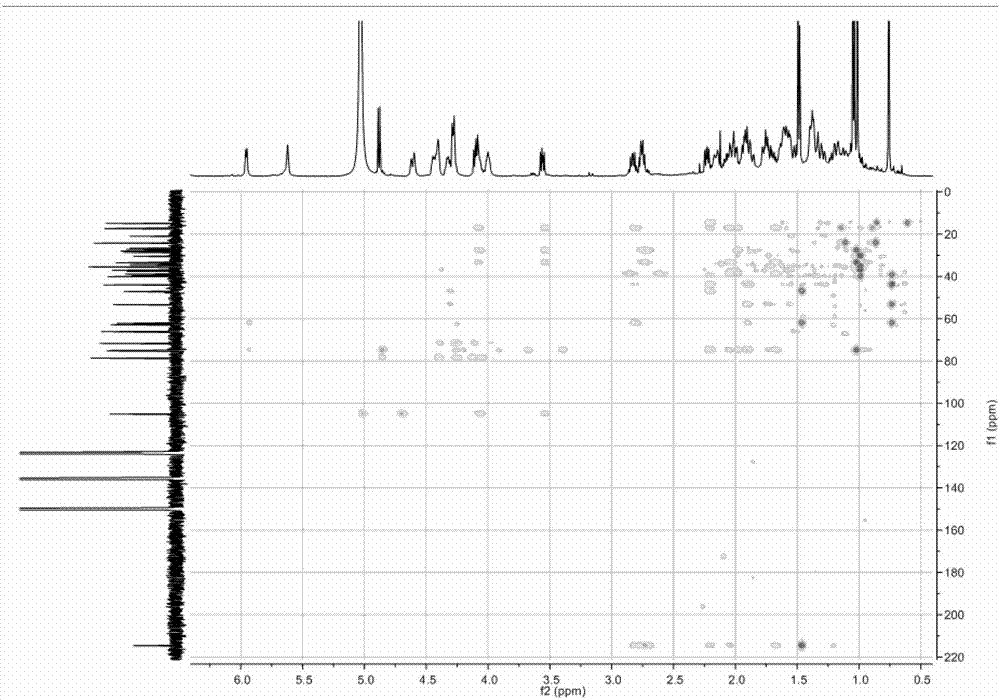
[0089] (3) Use 900mL-1200mL n-butanol to extract the solution obtained after neutralization in step (2) three times, and concentrate the extract under reduced pressure until evaporated to dryness to obtain furostane-type saponin monosaccharide derivative 2 (NMR: see Table 1 ).
[0090] (4) Dissolve the obtained furostane-type saponin monosaccharide derivative 2 solid in water, and make it ad...
Embodiment 2
[0092] Preparation of embodiment 2 furostane type saponin monosaccharide derivatives
[0093]
[0094] Using timosaponin B-Ⅲ as the reaction starting material to prepare furostane-type saponin monosaccharide derivatives, the specific steps are as follows:
[0095] (1) Dissolve 0.05mol timosaponin B-Ⅲ in 300ml methanol solution (water or 30v / v% ethanol solution or 1w / v% Tween solution) with a volume ratio of 10v / v%, add 2ml-4ml concentrated Hydrochloric acid, react at 80°C-150°C for 1 hour-6 hours.
[0096] (2) Adjust the above reaction solution to pH 6-7 with sodium hydroxide.
[0097] (3) Use 900ml-1200ml of n-butanol to extract the solution obtained after neutralization in step (2) three times, and concentrate the extract under reduced pressure until evaporated to dryness to obtain furostane-type saponin monosaccharide derivative 2 (NMR: see Table 1 for details) ).
[0098] (4) Dissolve the obtained furostane-type saponin monosaccharide derivative 2 solid with water, a...
Embodiment 3
[0100] Preparation of embodiment 3 furostane type saponin monosaccharide derivatives
[0101]
[0102]
[0103] Using timosaponin A Ⅰ as the reaction starting material to prepare furostane-type saponin monosaccharide derivatives, the steps are as follows:
[0104] (1) Dissolve 0.05mol timosaponin A I saponin 3 and 0.06mol-0.1mol tert-butyldimethylchlorosilane in 200ml-250ml DMF, and react at 60°C-90°C for 8 hours-10 hours; Add 600ml-800ml of petroleum ether to the reaction solution, then wash twice with 900ml water and once with 600ml NaCl aqueous solution. The organic phase was separated and dried over anhydrous sodium sulfate. After filtering, the filtrate was evaporated to remove the solvent under reduced pressure.
[0105] (2) Combine the product obtained in step (1) and 0.8mol-1.1mol NaHCO 3 Dissolve in 700ml dichloromethane, acetone, 1mmol / L Na at a ratio of 1:1:1 2 In the mixed solution of EDTA; Dissolve 1mmol / L-3mmol / L Na 2 Add EDTA solution to the reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com