Saponin derivative and application thereof
A technology of derivatives and saponins, which is applied in the application field of furostane-type saponin derivatives, prevention and treatment of depression, can solve the problems of no depression, few relationship between chemical structure and pharmacodynamic mechanism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
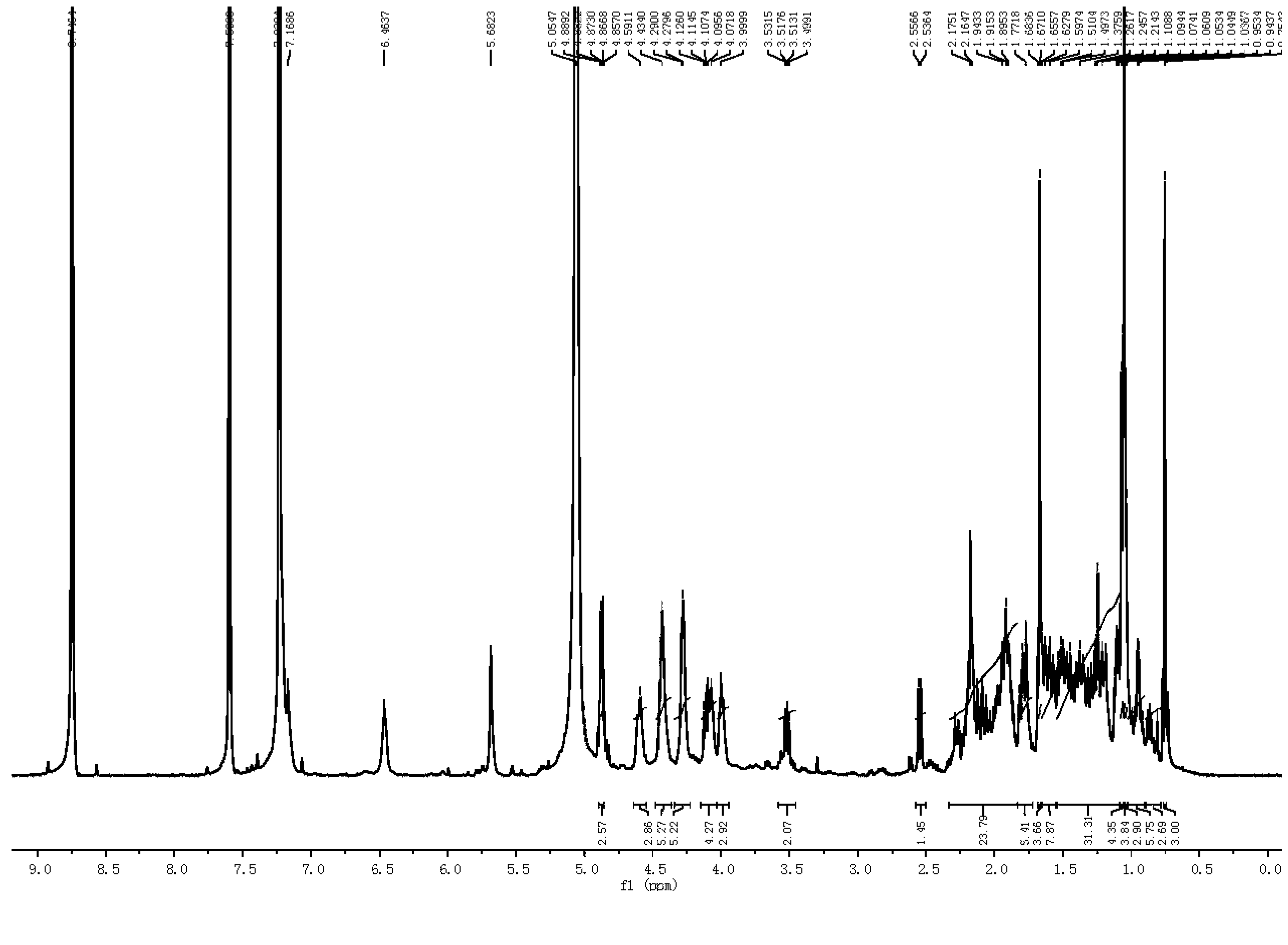
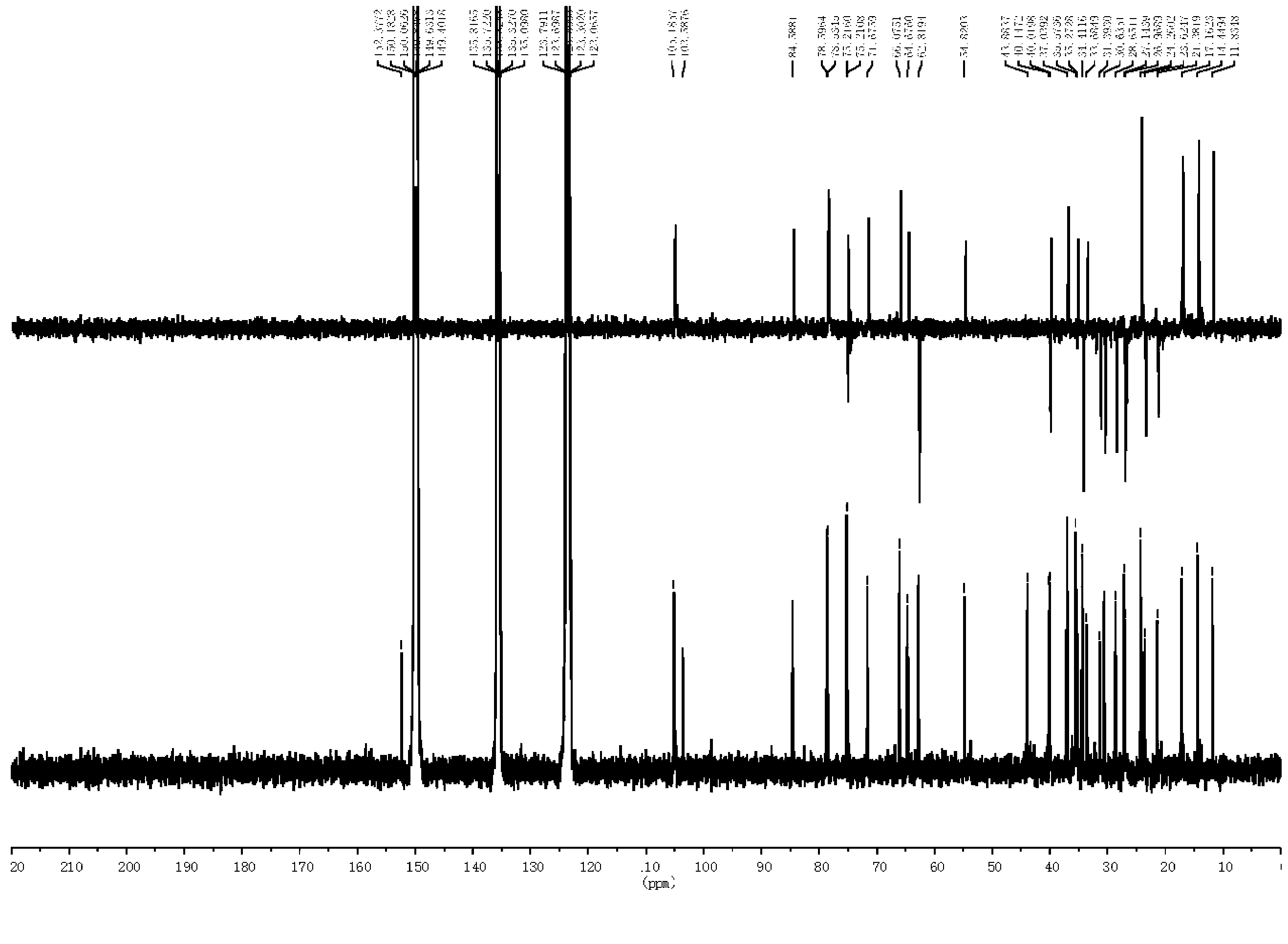
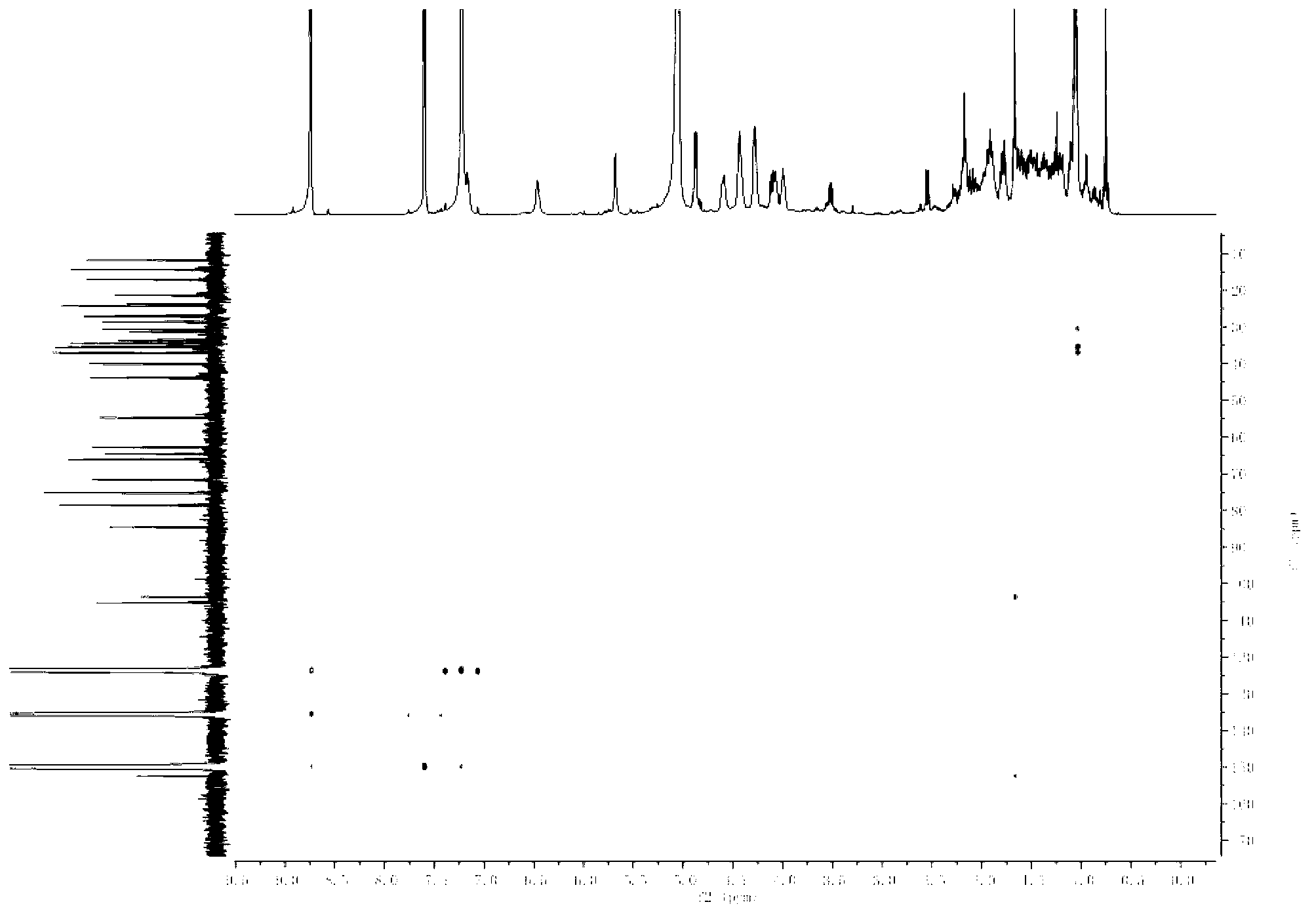
[0074] The preparation of embodiment 1 furostane type saponin monosaccharide derivative
[0075]
[0076] Using timosaponin B-Ⅲ as the reaction starting material to prepare furostane-type saponin monosaccharide derivatives, the specific steps are as follows:
[0077] (1) Dissolve 0.05mol timosaponin B-Ⅲ in 300ml methanol solution (water or 30v / v% ethanol solution or 1w / v% Tween solution) with a volume ratio of 10v / v%, and add 0.5ml-1ml Concentrated sulfuric acid, react at 80°C-150°C for 1 hour-6 hours.
[0078] (2) Adjust the pH of the above reaction solution to 6-7 with sodium hydroxide.
[0079] (3) Use 900ml-1200ml n-butanol to extract the solution obtained after neutralization in step (2) three times, and concentrate the extract under reduced pressure to evaporate to dryness.
[0080] (4) Dissolve the above solid with water, make the saponin adsorb on the ODS column, elute with 40v / v%-60v / v% methanol, and collect the eluate.
[0081] (5) The solution was recovered un...
Embodiment 2
[0082] Preparation of embodiment 2 furostane type saponin derivatives
[0083]
[0084] (1) Dissolve 0.05mol timosaponin B-III in 300mL methanol solution (water or 30% ethanol solution or 1% Tween solution) with a volume ratio of 10%, add 2mL-4mL concentrated hydrochloric acid, 80-150℃ React for 1 hour to 6 hours.
[0085] (2) Adjust the pH of the above reaction solution to 6-7 with sodium hydroxide.
[0086] (3) Use 900mL-1200mL n-butanol to extract the solution obtained after neutralization in step (2) three times, and concentrate the extract under reduced pressure until evaporated to dryness.
[0087] (4) Dissolve the above solid with water, make the saponin adsorb on the ODS column, elute with 40v / v%~60v / v% methanol, and collect the eluate.
[0088] (5) The solution was recovered under reduced pressure and evaporated to dryness to obtain furostane-type saponin monosaccharide derivative 2 (NMR: see Table 1 for details).
Embodiment 3
[0089] Preparation of embodiment 3 furostane type saponin monosaccharide derivatives
[0090]
[0091] Using timosaponin B-Ⅲ as the reaction starting material to prepare furostane-type saponin monosaccharide derivatives, the specific steps are as follows:
[0092] (1) Dissolve 0.1mol timosaponin B-III in 600ml methanol solution with a volume ratio of 10v / v%, add 30ml-50ml concentrated hydrochloric acid, and react at 80°C-100°C for 2 hours.
[0093] (2) Adjust to pH=6-7 with sodium hydroxide.
[0094] (3) Use 1.8L-2.4L n-butanol to extract the solution obtained after neutralization in step (2) three times, and concentrate the extract under reduced pressure until evaporated to dryness.
[0095] (4) Dissolve the above solid with water, make the saponin adsorb on the ODS column, elute with 40v / v%-60v / v% methanol, and collect the eluate.
[0096] (5) The solution was recovered under reduced pressure and evaporated to dryness to obtain aglycone 3 of mother saponin B-Ⅲ (NMR: 30....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com