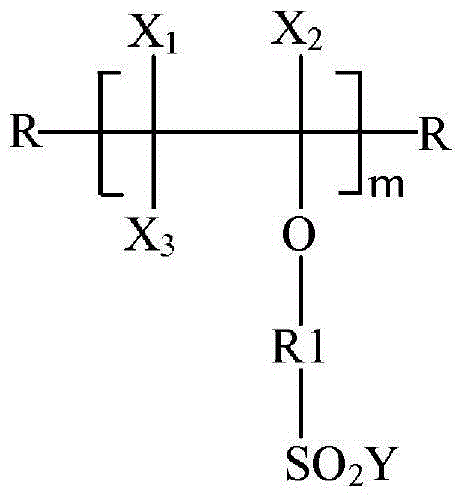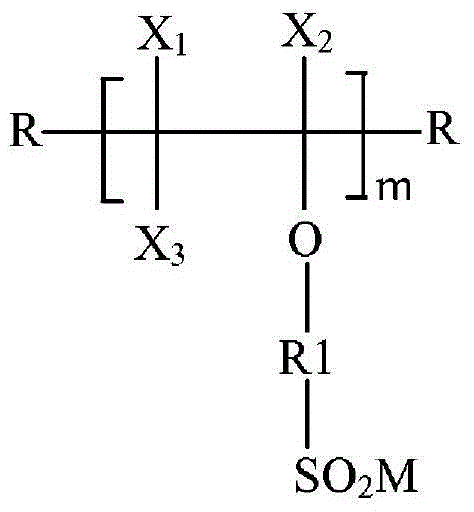Preparation of oligomers and co-oligomers of highly fluorinated sulfinic acids and their salts
A highly fluorinated, sulfinate technology for use in the field of sulfinic acid oligomers and co-oligomers and their salts, highly fluorinated sulfinic acid oligomers and co-oligomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Embodiment 1: a kind of method for preparing oligomer, described method comprises:
[0139] (a) providing a highly fluorinated vinylsulfonyl halide;
[0140] (b) oligomerizing the highly fluorinated vinylsulfonyl halide with an initiator to provide a highly fluorinated oligomeric sulfonyl halide according to the following formula (I):
[0141]
[0142] and (c), reducing the highly fluorinated oligomeric sulfonyl halide to a highly fluorinated sulfinate oligomer according to the following formula (IV),
[0143]
[0144] Among them, X 1 、X 2 and x 3 independently selected from F, Cl and CF 3 ; R is independently selected from H, I, Br, straight chain or branched chain alkyl and optionally a straight chain or branched chain fluoroalkyl group containing heteroatoms in the chain; R1 is straight chain or branched chain perfluorinated A linking group of Y, which may be saturated or unsaturated and substituted or unsubstituted, and optionally contains catenary hetero...
Embodiment 2
[0145] Embodiment 2: The method of embodiment 1 further comprising (d): acidifying the highly fluorinated sulfinate oligomer from step (c) and extracting the highly fluorinated sulfinate therefrom acid oligomers.
Embodiment 3
[0146] Embodiment 3: The method of embodiment 2, further comprising (e): converting the highly fluorinated sulfinic acid oligomer from step (d) to form a salt thereof.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com