Antimicrobial mixture of aldehydes, organic acids and organic acid esters
An organic acid ester, antimicrobial technology, used in food ingredients as antimicrobial preservation, chemicals for biological control, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
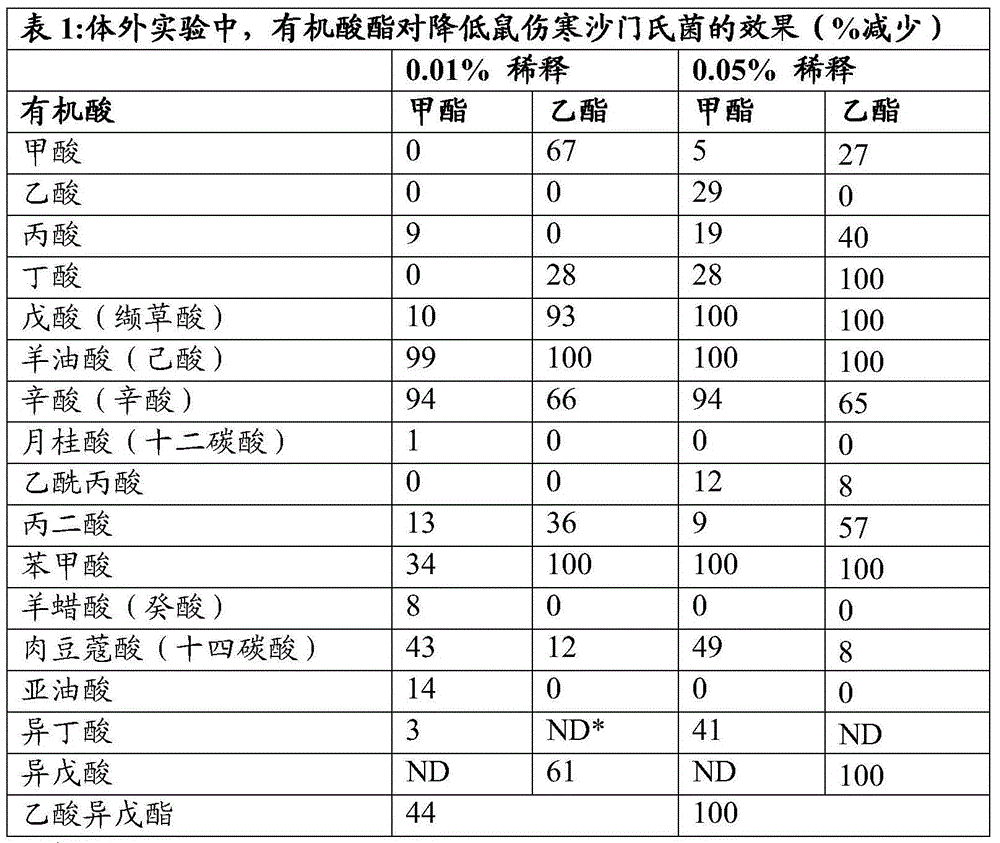
[0112] Organic acid methyl esters and organic acid ethyl esters were added to the test tubes at concentrations of 0.01% and 0.05%. The tube was vortexed for 10 seconds to mix the solution evenly. A suspension of Salmonella typhimurium (ATCC: 14028) was added to each test tube to obtain 10 4 Final concentration of cfu / ml. The solution was vortexed, then incubated at room temperature for 24 hours, and plated onto XLT-4 agar. Plates were incubated at 37°C for 48 hours before colonies were counted. The potency of each ester is shown in Table 1 below as a percent reduction compared to the control value.
[0113]
[0114] *ND not determined
[0115] Observed: chain length C 4 ~C 8 Esters of organic acids were effective against Salmonella at the concentrations tested. Ethyl esters are generally more effective than methyl esters. Esters of benzoic acid (aromatic cyclic acid) and isoamyl acetate (isoamyl ester of acetic acid) were also observed to have fungicidal activity. ...
Embodiment 2
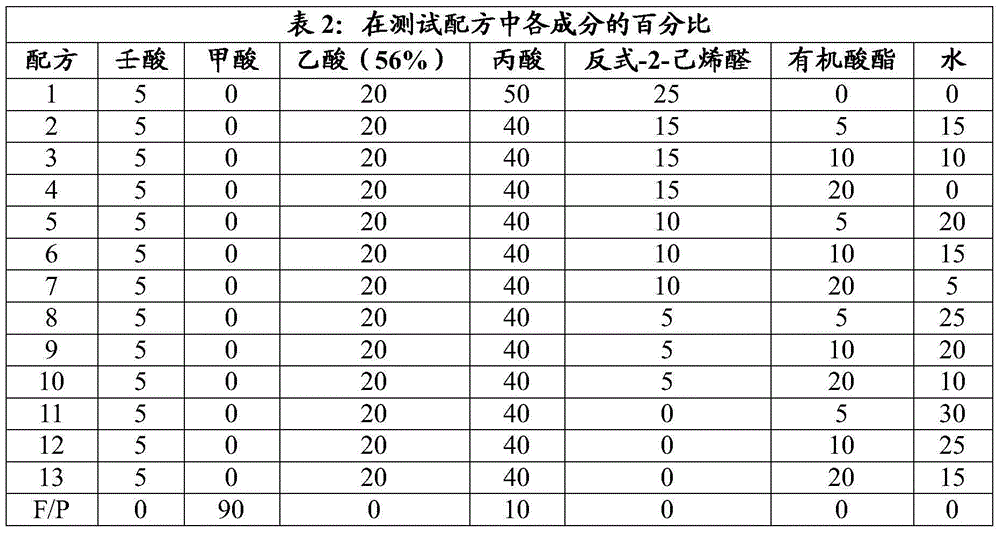
[0117] As shown in Table 2 below, eight kinds of organic acid esters (C 4 ~C 8 organic acid esters and benzoates) mixed with trans-2-hexenal, nonanoic acid, propionic acid, acetic acid and water. A 25% hexanal:organic acid product (Recipe 1) and formic acid:propionic acid (90:10, F / P) product were included as positive controls. The formulations were added to test tubes at concentrations of 0.01% and 0.005%. The tubes were vortexed for 10 seconds to mix the solution evenly.
[0118]
[0119] Salmonella typhimurium (10 4 cfu / ml) to tubes containing different dilutions of each formulation. The solution was vortexed, then incubated at room temperature for 24 hours, and plated onto XLT-4 agar. Plates were incubated at 37°C for 48 hours before colonies were counted.
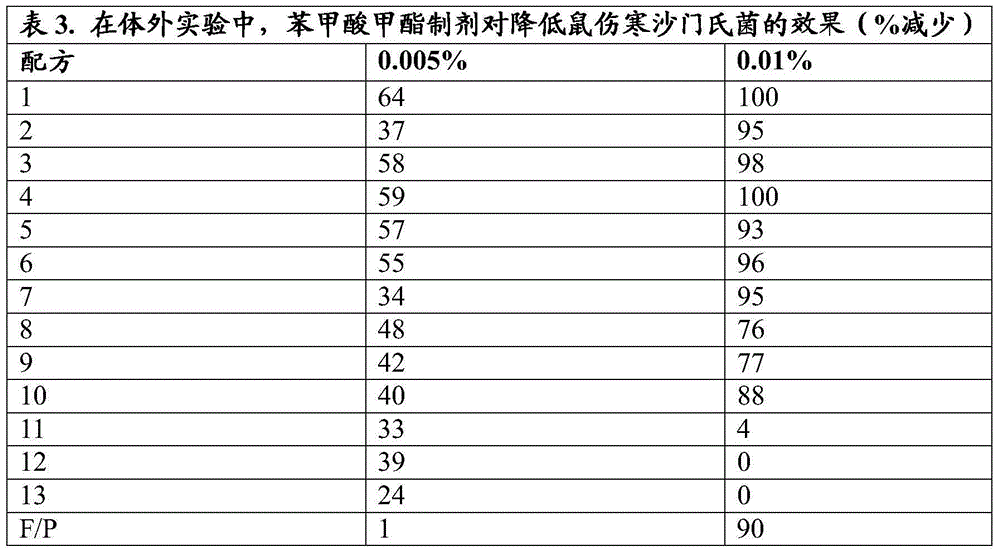
[0120] The potency of each formulation is shown in Tables 3 to 10 below as a percent reduction compared to the control value.
[0121]
[0122]
[0123]
[0124]
[0125]
[0126]
[0127] ...
Embodiment 3
[0133] Eighteen formulations were prepared for the in vitro studies shown in Table 11. 25% trans-2-hexenal:organic acid product (Recipe 1) and formic acid:propionic acid (90:10, F / P) product were included as positive controls. The formulations were added to test tubes at concentrations of 0.005% and 0.01%. The tube was vortexed for 10 seconds to mix the solution evenly.
[0134]
[0135] Salmonella typhimurium (10 4 cfu / ml) to tubes containing different dilutions of each formulation. The solution was vortexed, then incubated at room temperature for 24 hours, and plated onto XLT-4 agar. Plates were incubated at 37°C for 48 hours before colonies were counted. The potency of each formulation is shown in Table 12 below as a percent reduction compared to the control value.
[0136]
[0137]
[0138] Addition of 5% of each ester to an organic acid product containing 20% trans-2-hexenal was equivalent in efficacy to an organic acid product containing 25% trans-2-hexen...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap