8-Carbamoyl-2-(2,3-disubstituted pyridin-6-yl)-1,2,3,4-tetrafluoroethylene as an apoptosis-inducing agent for the treatment of cancer and immune and autoimmune diseases Hydroisoquinoline Derivatives
A pyridyl and substituent technology, applied in the field of diseases expressing anti-apoptotic Bcl-xL protein during the treatment of diseases, can solve problems such as weak binding only
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
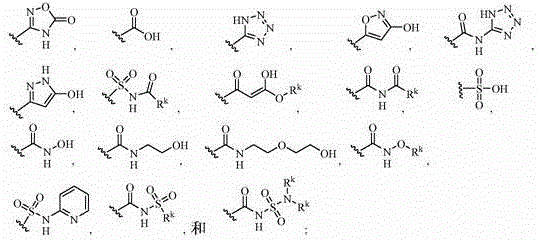
[0945] After preparation as described in Scheme 1, a compound of formula (6) can be combined with a boronic acid (or boronate equivalent) of formula (8) or wherein Y 1 , L 1 and Y 2 Organotin or organozinc halides of formula (8a) as described herein and M is tributyltin or zinc halide are reacted under Suzuki, Stille or Negishi coupling conditions known to those skilled in the art and readily available in the literature to give Compounds of formula (I) are provided. Alternatively, compounds of formula (7), which can be prepared from compounds of formula (6) as described in Scheme 1, can be combined with 1 is a triflate or halide and Y 1 , L 1 and Y 2 Compounds of formula (9) as described herein are reacted under Suzuki coupling conditions known to those skilled in the art and readily available in the literature to provide compounds of formula (I).
[0946]
[0947] As shown in Scheme 3, where R x1 is hydrogen or Y as described herein 1 The pyrazole of the formula (1...
Embodiment 1
[0963] 6-[8-(1,3-Benzothiazol-2-ylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl]-3-{1-[tricyclo[3.3 .1.1 3,7 ]dec-1-ylmethyl]-1H-pyrazol-4-yl}pyridine-2-carboxylic acid
Embodiment 1A
[0965] 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1-(tricyclo[3.3.1.1 3,7 ]dec-1-ylmethyl)-1H-pyrazole
[0966] 1-(Bromomethyl)adamantane (0.458 g) and 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- A mixture of 1H-pyrazole (0.377g) in N,N-dimethylformamide (5ml) was cooled to 0°C. To this solution was added 60% sodium hydride (0.096 g). The solution was heated at 70°C overnight. The reaction mixture was partitioned between water and ethyl acetate. The aqueous layer was extracted twice with additional ethyl acetate. The combined organic layers were washed with brine, washed with MgSO 4 Dry, filter and concentrate. The residue was purified by flash column chromatography on silica gel eluting with 25% ethyl acetate / hexanes to provide the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com