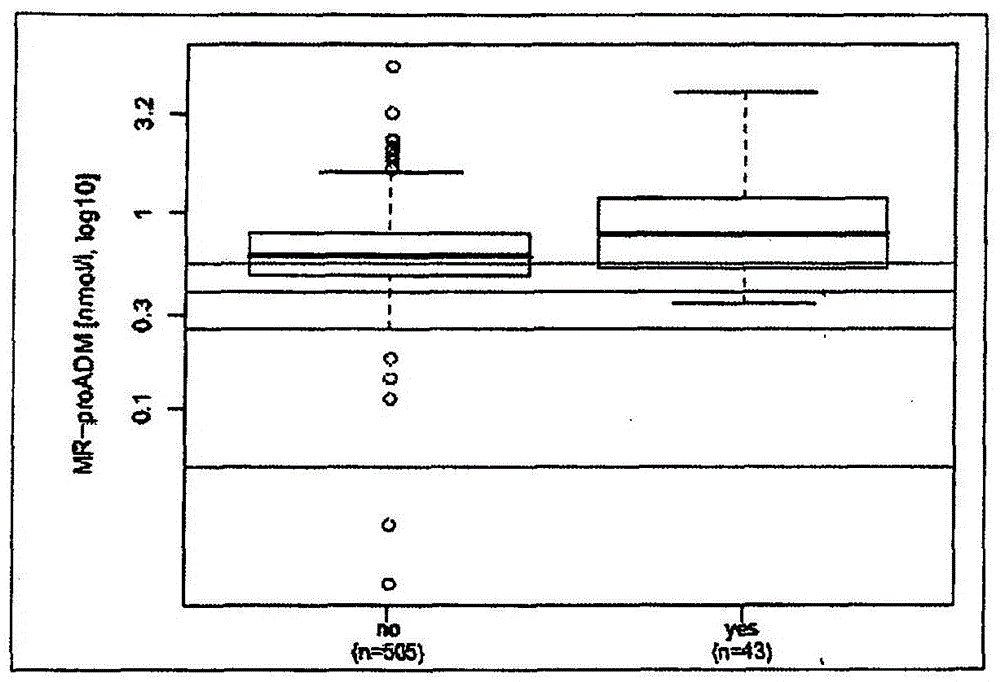
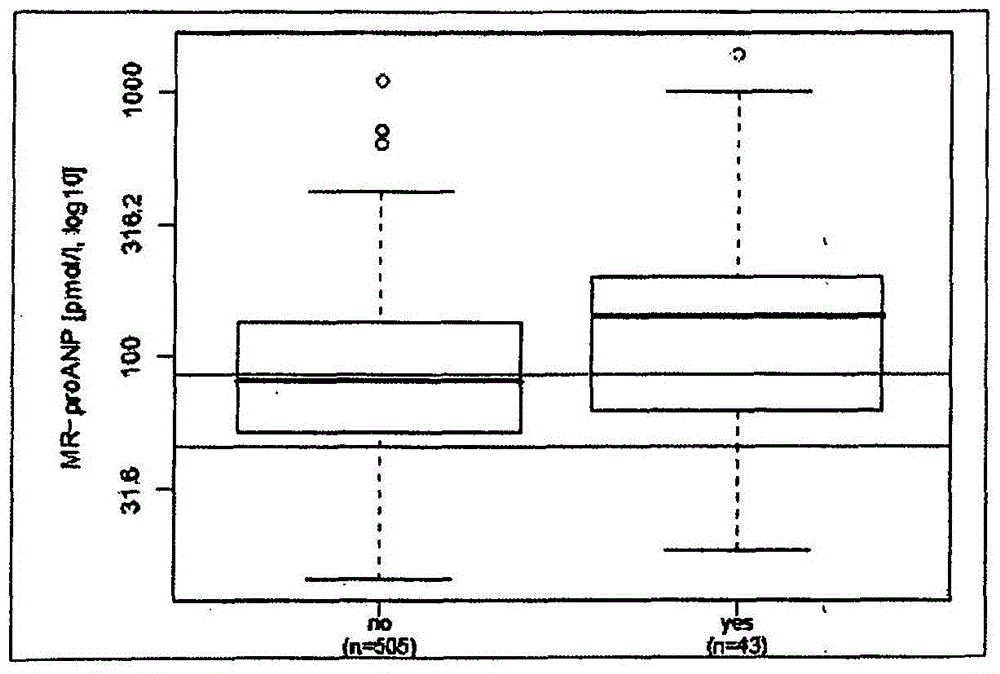
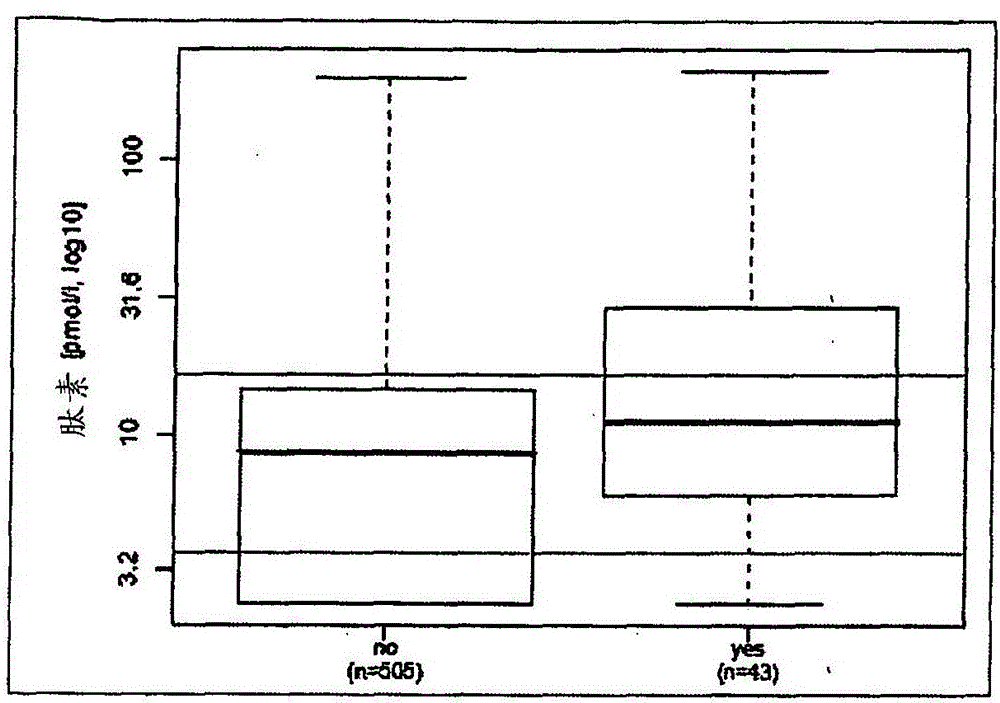
Prediction of outcome in patients with chronic obstructive pulmonary disease
A patient and prognostic technology, applied in the field of clinical diagnostics, can solve the problems of unknown prognostic value of biomarkers, lack of risk stratification strategy for ambulatory patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0125] marker measurement
[0126] MR-proANP was detected using a novel fully automated sandwich immunoassay system on the B.R.A.H.M.S KRYPTOR (B.R.A.H.M.S GmbH, Hennigsdorf / Berlin, Germany). The stochastic on-line analyzer uses a sensitive Time Resolved Amplified Cryptate Emission (TRACE) technique based on the non-radioactive transfer between 2 fluorophores, europium cryptate and XL665. This automated assay is essentially based on a sandwich chemiluminescent assay described in detail elsewhere ( Morgenthaler et al 2004.Clin Chem50:234-6 ), and for other studies ( Khan et al. 2008. J Am Coll Cardiol 51:1857-64; Gegenhuber et al. 2006. Clin Chem 52: 827-31 ). For MR-proANP detection, 14 μl of patient serum was incubated for 14 min. The measurement range is 0-10000pmol / L, the detection limit is 2.1pmol / L, and the quantification limit is 4.5pmol / L. The intra-assay CV was 1.2% and the inter-laboratory CV was 5.4%. This assay uses the same antibody pair as the reference...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com