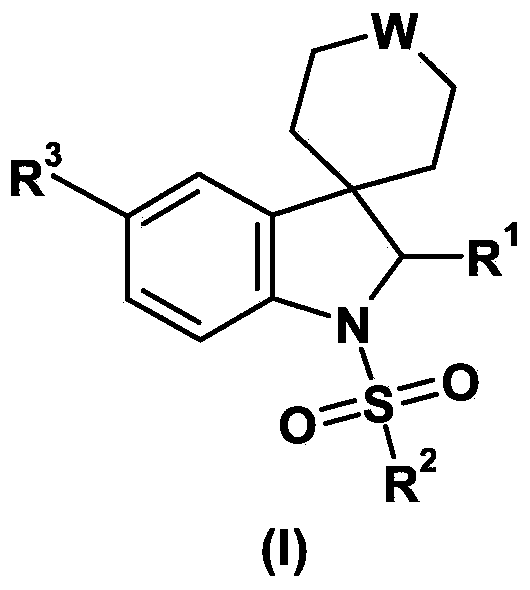
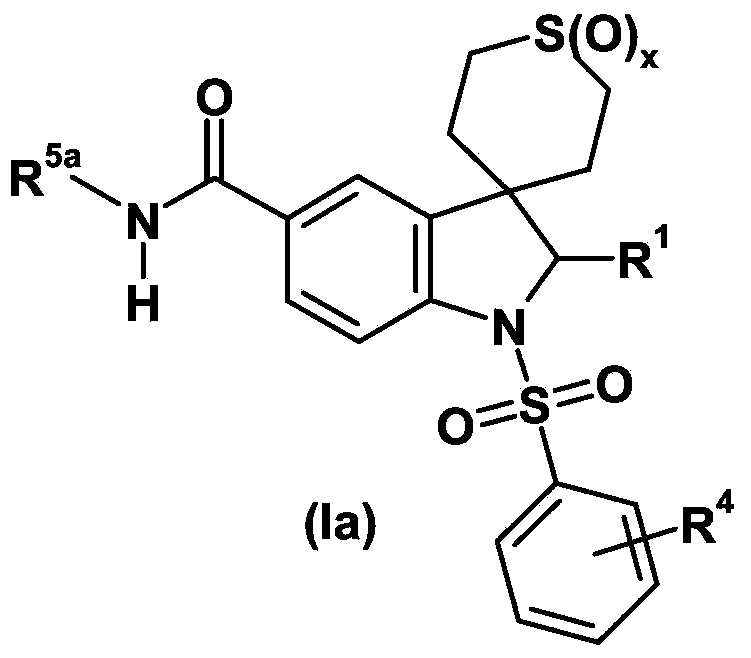
Spiroindoline derivatives as gonadotropin-releasing hormone receptor antagonists
A technology of compound and alkyl, which is applied in the field of disease treatment, can solve the problem that the activity of GnRH receptor antagonist is not described, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0602] N-[(3-chloropyridin-2-yl)methyl]-1-[(4-fluorophenyl)sulfonyl]-2-methyl-1,2,2',3',5',6 ′-Hexahydrospiro[indole-3,4′-pyran]-5-carboxamide
[0603]
[0604] According to GP 9.1, 250 mg (0.62 mmol) of intermediate F.3 and 105 mg (0.74 mmol, 1.2 eq) of 1-(3-chloro Pyridin-2-yl)methanamine (CAS No. [500305-98-6]) was reacted with 280 mg (0.74 mmol, 1.2 equiv) of HATU in 10 mL DMF to yield 300 mg (84%) of the desired amide. 1 H-NMR (300MHz, DMSO-d6): chemical shift [ppm]=-0.02(d, 1H), 0.97-1.08(m, 1H), 1.25(d, 3H), 1.67(d, 1H), 1.96- 2.08(m, 1H), 3.32-3.50(m, 3H), 3.79-3.87(m, 1H), 4.50(q, 1H), 4.63(d, 2H), 7.33(dd, 1H), 7.35-7.41( m, 2H), 7.54 (d, 1H), 7.76 (d, 1H), 7.81 (dd, 1H), 7.87-7.92 (m, 3H), 8.44 (dd, 1H), 8.80 (t, 1H). UPLC-MS(ESI+): [M+H] + =530 / 532 (chlorine isotope pattern).
[0605] The enantiomers of the racemic material of Example 1 were analyzed by chiral preparative HPLC (System: Dionex: Pump P 580, Gilson: Liquid Handler 215) , Nuoer Company (Knauer...
Embodiment 11
[0606] Example 1.1: R t =17.81min;
Embodiment 12
[0607] Example 1.2: R t =23.01min;
[0608] Table 1 Applying the indicated general procedures, the following examples (3 to 11) were prepared in analogy to example 1 starting from intermediate F.3 and commercially available amines. Example 2 was prepared from intermediate C.5 according to the given procedure.
[0609]
[0610]
[0611]
[0612] Example 12
[0613] N-(2-chlorobenzyl)-2-cyclopropyl-1-[(4-fluorophenyl)sulfonyl]-1,2,2',3',5',6'-hexahydrospiro[ Indole-3,4′-thiopyran]-5-carboxamide
[0614]
[0615] With GP 10: Dissolve 2.70 g (5.60 mmol) of intermediate C.1 in 40 mL 1,4-dioxane (containing 0.1 mL water) and add 2.38 g (3 equiv.) of 2-chlorobenzyl Amine (CAS No. [89-97-4]), 1.48 g (1 equivalent) of molybdenum hexacarbonyl, 1.78 g (3 equivalents) of sodium carbonate, 162 mg (0.1 equivalent) of tri-tert-butylphosphonium tetrafluoroborate and 126 mg (0.1 equivalent) palladium(II) acetate. The mixture was heated to reflux for 18h (bath temperature wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com