CRISPR/Cas9 recombinant lentiviral vector and its lentivirus for AIDS gene therapy
A technology of recombinant lentivirus and lentiviral vector, which is applied in the field of medicine and genetic engineering, can solve the problem of high off-target rate, achieve the effect of inhibiting the spread of infection and delaying invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
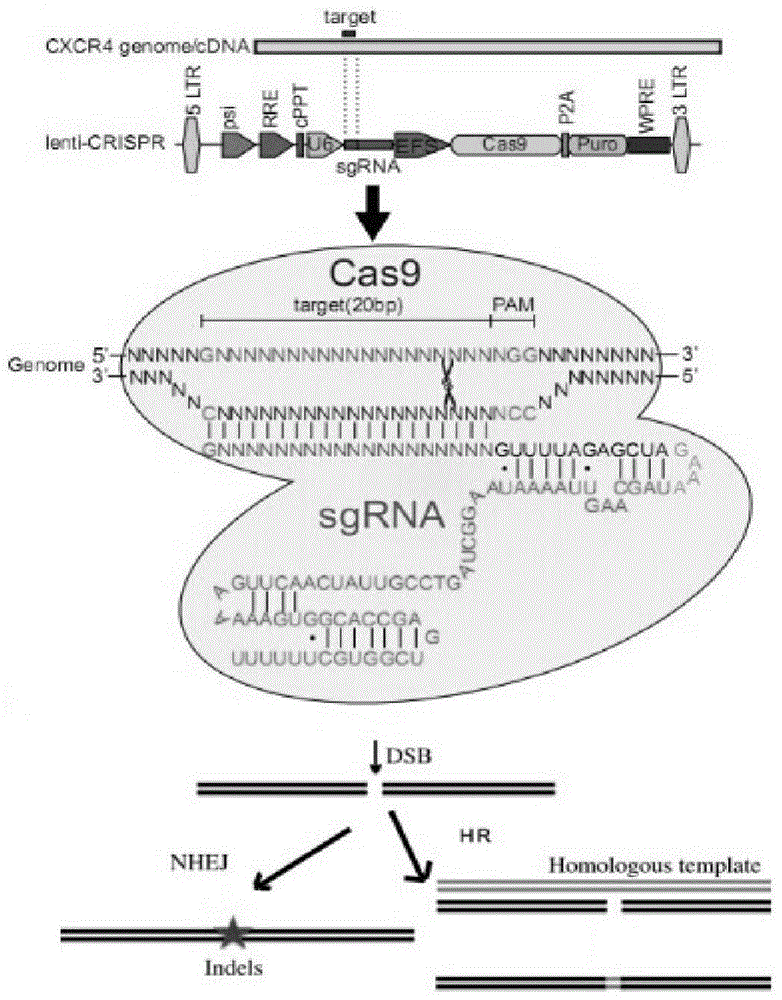
[0060] [Example 1] Construction of a CRISPR / Cas9 recombinant lentiviral vector containing a specific target sequence of CXCR4 1, Conservation analysis of the target sequence targeting the HIV co-receptor CXCR4 gene
[0061] In order to clarify the conservation of the CXCR4 target sequence selected by the applicant in humans and monkeys, the applicant applied the BLAST software in the National Center For Biotechnology Information Database to analyze the sequence conservation of the CXCR4 gene in humans and rhesus monkeys. In the conserved exon region, select the target sequence that conforms to the characteristics of CRISPR-Cas9 binding genome [5'-G(19N)-NGG3' or 5'-CCN(19N)C-3']. We designed 10 target sites (Table 1), and all target site sequences were completely identical (100%) between humans and monkeys. 2. Construction of recombinant lenti-CRISPR and identification of gene editing effects
[0062] The modified lentiviral vector is pLentiCRISPR (Addgene plasma ID: 52961), ...
Embodiment 2
[0092] [Example 2] Packaging of CRISPR / Cas9 lentiviral system
[0093] In order to further improve the gene knockout efficiency of the CRISPR / Cas9 recombinant lentiviral vector, the CRISPR / Cas9 recombinant lentiviral vector was packaged into lentivirus in HEK-293T cells.
[0094] The lentiviral packaging vector is:
[0095] lentiCRISPR vectors for different targets
[0096] psPAX2 lentiviral helper packaging plasmid (purchased from Addgene)
[0097]pMD2.G lentiviral helper envelope plasmid (purchased from Addgene)
[0098] The specific process is as follows:
[0099] 1) HEK-293T cells (purchased from CCTCC) were plated in a cell culture dish with a diameter of 10 cm 24 hours before transfection.
[0100] 2) The confluence of the cells should be 80-90% during transfection. The above plasmids were co-transfected into the cells with the standard Lipofectamine2000 transfection reagent, and the medium was changed 6 hours after transfection.
[0101] Transfection system:
[01...
Embodiment 3
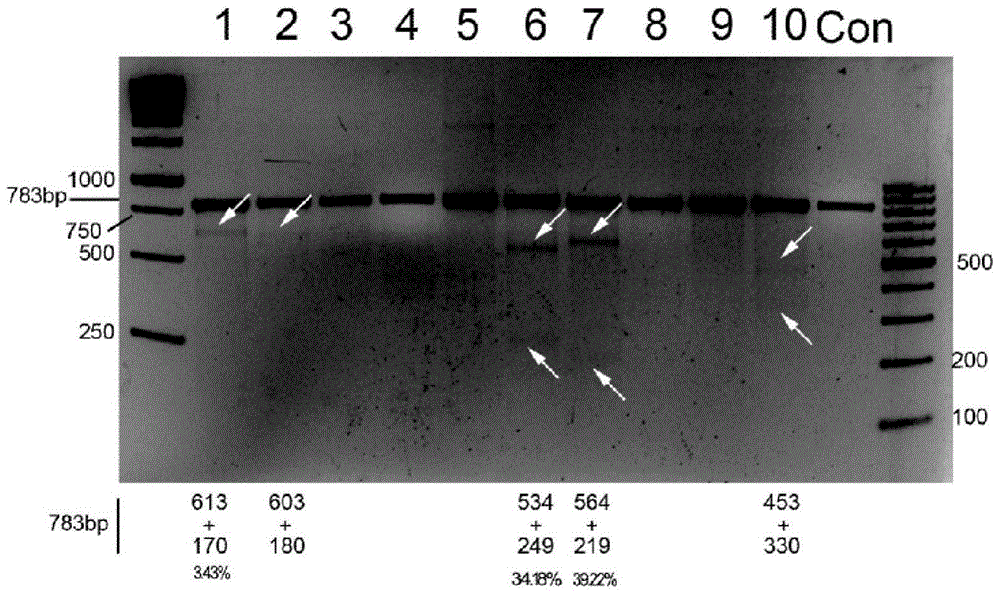
[0106] [Example 3] Using CRISPR / Cas9 recombinant lentivirus to establish a cell line that stably knocks out the CXCR4 gene 1) Infect with the lentivirus packaged in Example 2 with an m.o.i. of 100 (1ml virus supernatant added to 4ml DMEM cells) GhostCD4 / CXCR4 cells, the ratio of CXCR4 positive cells detected by flow cytometry after 48 hours, the PE-CXCR4 positive cell rate of the unmodified Con cells was 87.1%, while the PE-CXCR4 positive cell rate of the lentiCRISPR6# and 7# modified cells 23.5% and 29.5%, respectively. Compared with the control, they were down-regulated by 73.02% and 66.1% ( Figure 4 ).
[0107] 2) A small portion of cells were collected for Western blot assay to detect the expression level of CXCR4 ( Figure 5 );
[0108] 3) The rest of the cells were sorted by flow cytometry with PE-CXCR4 antibody, and the purity of the stable cell line was tested after sorting, the results are shown in Image 6 , subculture the sorted cells to an appropriate cell num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com