Anti-hsv gb monoclonal antibody or antigen-binding fragment thereof
A technology of monoclonal antibodies and binding fragments, applied in the direction of antibodies, antiviral agents, hybrid immunoglobulins, etc., can solve the problems of non-existence of vaccines, increase of drug-resistant virus strains, withdrawal of drugs, etc., to prevent the activation of HSV infection, The effect of treating HSV infection and inhibiting the spread of infection between cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] The making of embodiment 1 anti-HSV gB monoclonal antibody D48
[0106] Production of scFv-hFc type, Fab type, human-mouse chimeric IgG2a type, and human-guinea pig chimeric IgG2κ for antibody D48 obtained by exhaustive search of anti-HSV-2gB (HSV gB2) antibodies using a human antibody library Antibody D48 of these four molecular forms was further analyzed.
[0107]
[0108] The isolated antibody D48 scFv (single chain Fv, single chain antibody) gene encoding the amino acid sequence shown in SEQ ID NO: 9 was linked to the Fc gene (CH2-CH3) from human IgG1, and cloned into pCAG vector to construct scFv- Fc expression plasmid. For expression, FreeStyle293 or Expi293 expression system (Life Technology Co., Ltd.) was used. The expression plasmid was transfected into the cells, and the culture supernatant was collected after 4-6 days. The culture supernatant was purified with Ab-Rapid PuRe 10 (ProteNova) or Ab-Rapid PuRe Ex (ProteNova) to obtain scFv-hFc.
[0109]
...
Embodiment 2
[0115] Embodiment 2 utilizes the reactivity analysis of the anti-HSV gB antibody of ELISA
[0116]
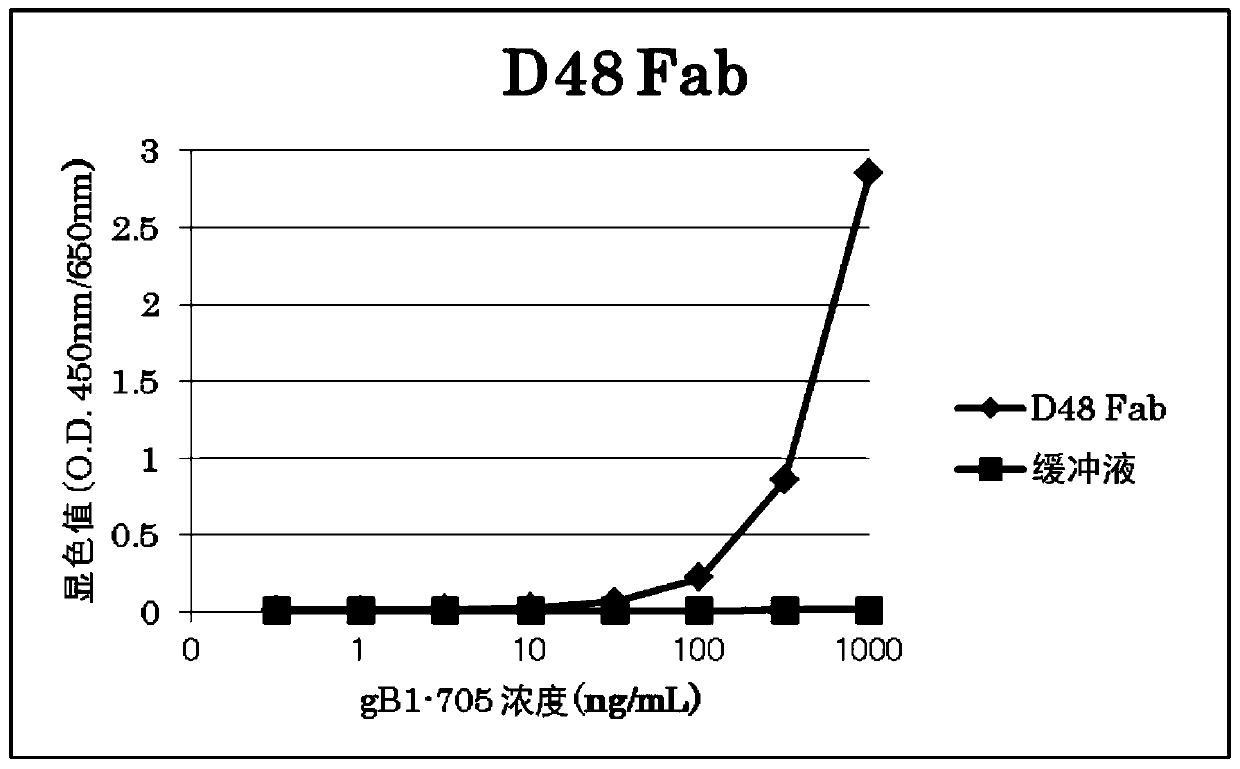
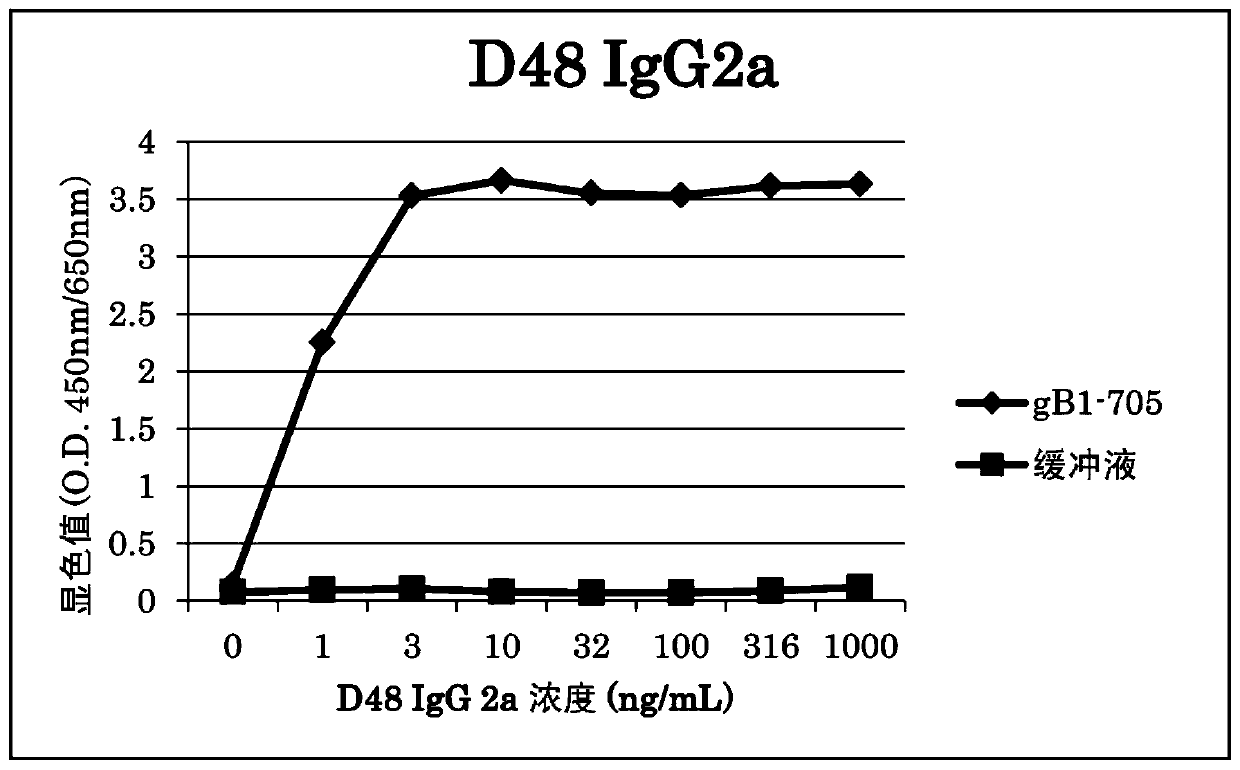
[0117] The binding activity of the obtained Fab was evaluated by ELISA. Fab was diluted to 2 μg / mL with PBS, 100 μL was added to MaxiSorp plate (Nunc), and incubated at room temperature for 2 hours to immobilize Fab. After immobilization, wash the plate with PBS, serially dilute from 1 μg / mL to 0.316 ng / mL by 3.16 times, add 100 μL to the wells of the recombinant HSV-1gB (gB1-705-strep) plate, and incubate at 37°C. After washing with PBST for 1 hour, 100 µL of detection antibody anti-strep-Tactin (strep-Tactin) / HRP (Funakoshi) was added to the wells of the plate, and incubated at 37°C. After washing with PBST for 1 hour, 100 µL of TMB (3,3',5,5'-tetramethylbenzidine) was added to the wells of the plate to develop color. After 30 minutes, the reaction was terminated with 1N sulfuric acid, and the color development value (O.D.450nm / 650nm) was measured with a microplate reader...
Embodiment 3
[0122] Embodiment 3 utilizes the reactivity analysis of the anti-HSV gB antibody of immunoblotting
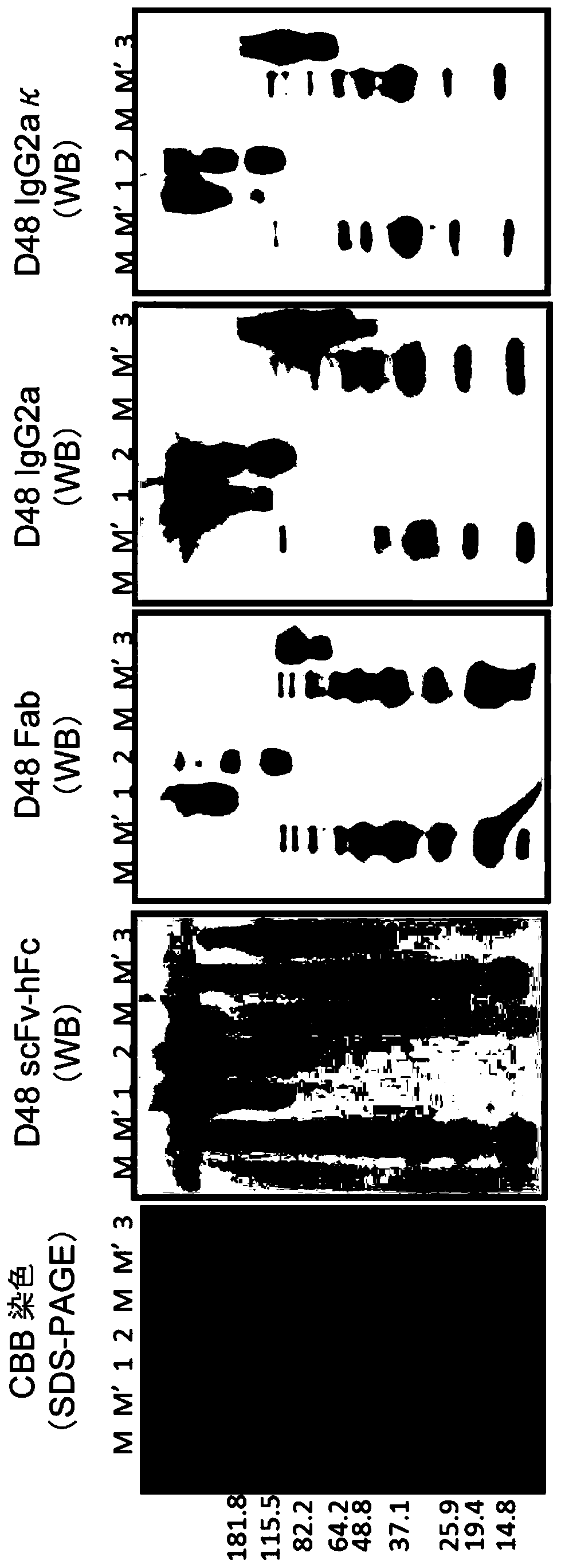
[0123] 2 μg / lane of gB1-705-strep was injected into an 8-16% by weight gel for SDS-PAGE, and electrophoresis was performed. After electrophoresis, the gel was transferred to a nitrocellulose membrane (Immobilon-P, MILLIPORE), and blocked with 2% skim milk (Wako)-PBST. After the blocked nitrocellulose membrane was washed with PBST, react with 2% skim milk-PBST, and 10 μg / mL scFv-hFc, Fab, human-mouse chimeric IgG2a or human-guinea pig chimeric IgG2κ at room temperature for 60 minute. After washing again, the nitrocellulose membrane was mixed with anti-hIgG (H+L) / HRP (BIORAD), anti-His tag / HRP (R&D), anti-mouse IgG (H+L) in 2% skim milk-PBST, respectively. / HRP or anti-guinea pig (H+L) / HRP (Invitrogen) reaction, developed with Immobilon Western Detection Regent (Millipore). Produce undenatured gB1-705-strep, denatured gB1-705-strep, and reduced / denatured gB1-705-strep. Reduct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com