Method for quantifying immune cells in tumoral tissues and its applications
A technology of immune cells and tumor tissues, applied in measuring devices, instruments, biomaterial analysis, etc., can solve problems such as parameters that do not prove the spatial heterogeneity of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Example 1: Preparation of stained slides of tumor tissue
[0169] Two tissue paraffin sections of selected 4 micron tumor masses were prepared and stored in deionized water on microscope slides (Superfrost-plus slides) for immunohistochemistry. Tissue paraffin sections were dried at room temperature and incubated overnight in an oven at 56°C-58°C.
[0170] Immunostaining was performed with IVD certified antibodies against CD3 and CD8 (CONFIRM CD3 (2GV6, Ventana) and CD8 (C8 / 144B; Dako)). The associated protocol contains the critical steps of blocking, epitope retrieval, and detection. Modified Mayer's hematoxylin (Hematoxillin II, Ventana) intended for staining nuclei on slides was applied for optimal detection of stained cells with dedicated software. The solution used with Benchmark XT automation (Roche-Ventana) is:
[0171]
[0172] CC1 is a tris-based buffer with a slightly basic pH. The primary antibody diluent against CD8 was K004 (Clinisciences).
[0173]...
Embodiment 2
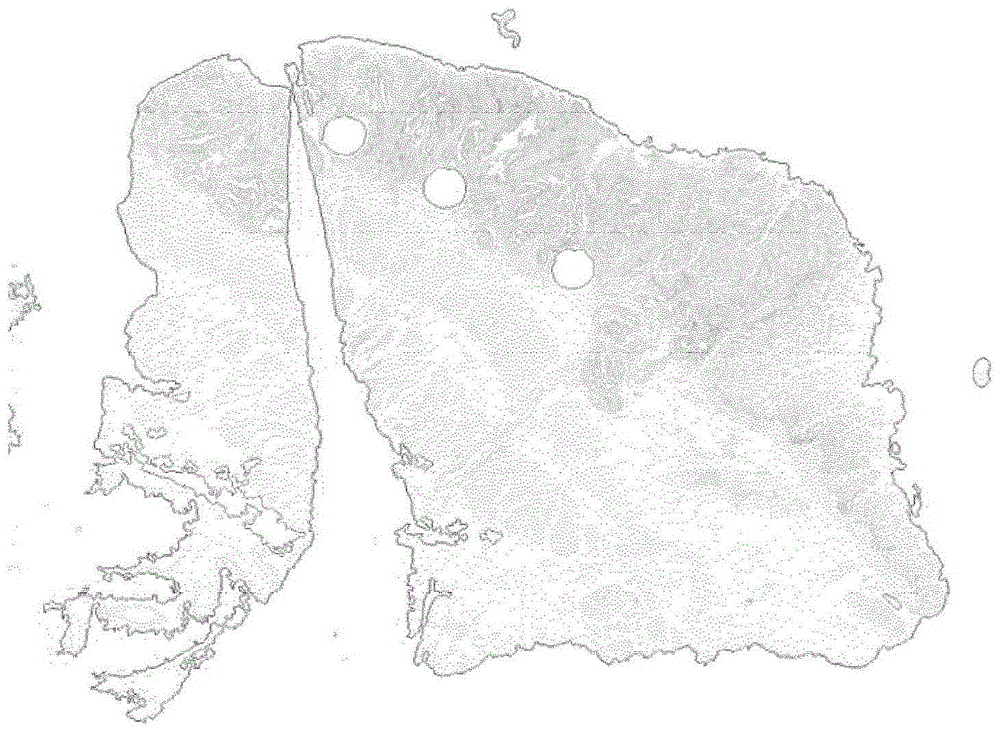
[0175] Example 2: Analysis of stained slides by software processing
[0176] .The pathologist has uploaded the digital images of each immunohistochemistry for CD3 and CD8 and started the analysis with the dedicated image analysis software (Definiens Developer XD).
[0177] .The semi-automatic procedure consists of steps for:
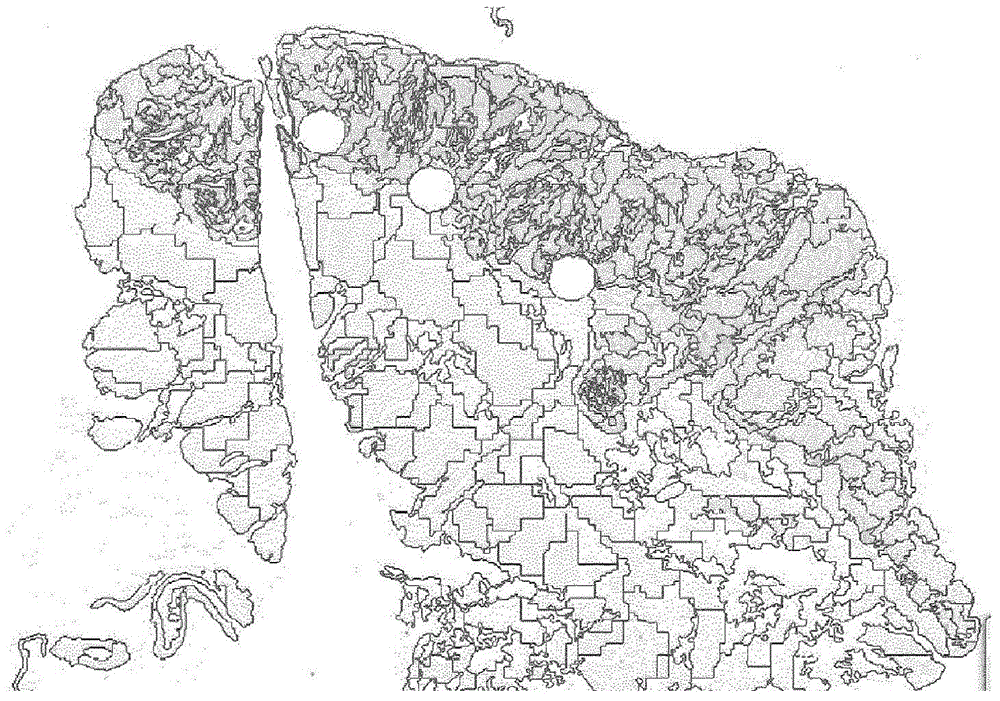
[0178] - automatic detection of tissues,
[0179] - Automatic segmentation of tissue into units,
[0180] - manual removal of artificial parts (folds, tears, air bubbles, ...),
[0181] - manual selection of tumor areas by the pathologist using a brush as a digital tool,
[0182] - automatic detection of intrusive edges,
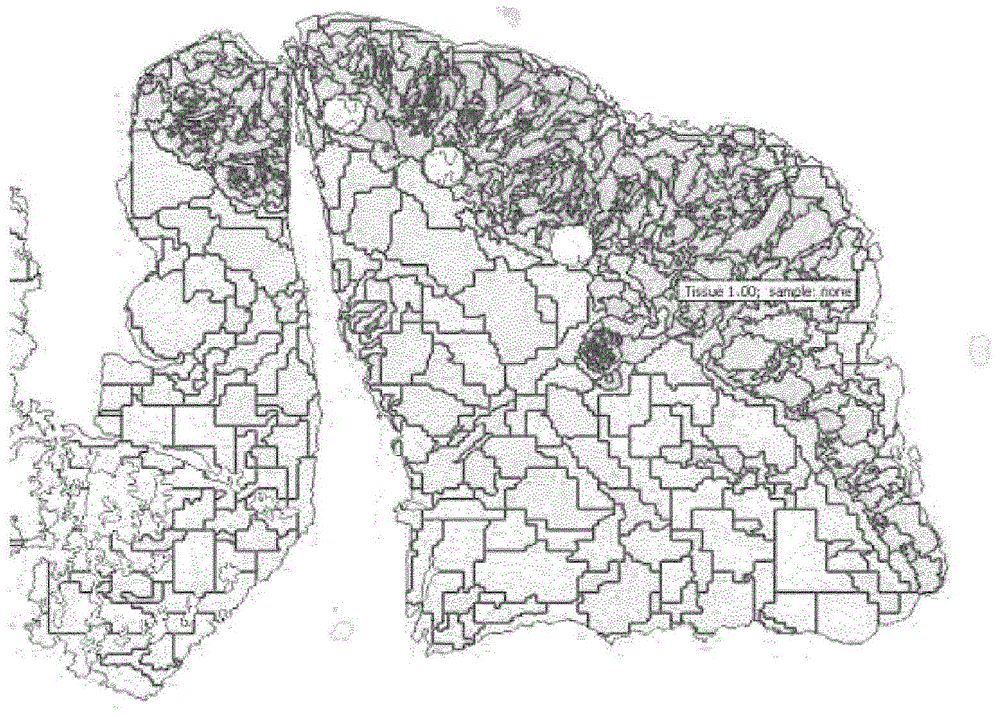
[0183] - automatic detection of stained cells in each cell of the tumor,
[0184] - Analysis of the distribution, mean and median of the staining intensity of the positive cells detected by the software in the graph to verify the immunostaining and quantification of the stained cells; this analysis is carried out for each cell of ...
Embodiment 3
[0202] Example 3: Example of Calculation of Optimal Threshold Values from P-Values (Log-Rank Test) of Associated Disease-Free Survival Rates
[0203] Calculate p-value for disease-free survival (log-rank test)
[0204] For 20 to 2000 CD3+ cells / mm 2 For each value of , the number of patients with a density of CD3+ cells in the tumor (CT region) (dots on the bottom curve) less than the value was determined (thus providing the patient group).
[0205] Calculation of 20 to 2000 CD3+ cells / mm in tumors (dots and corresponding curves) comparing patient groups 2 The p-value of the log-rank test for each threshold of .
[0206] result in Figure 15 available in .
[0207] X-axis represents cells / mm 2 Cell densities are indicated, and the Y-axis indicates log-rank p-values (dots and corresponding curves at the bottom of the graph). Hazard ratios (square points and corresponding curves) and iAUC concordance index (thin curves) are also represented.
[0208] Patients were c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com