anti-alk2 antibody
An antibody and antigen technology, applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., can solve unknown problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
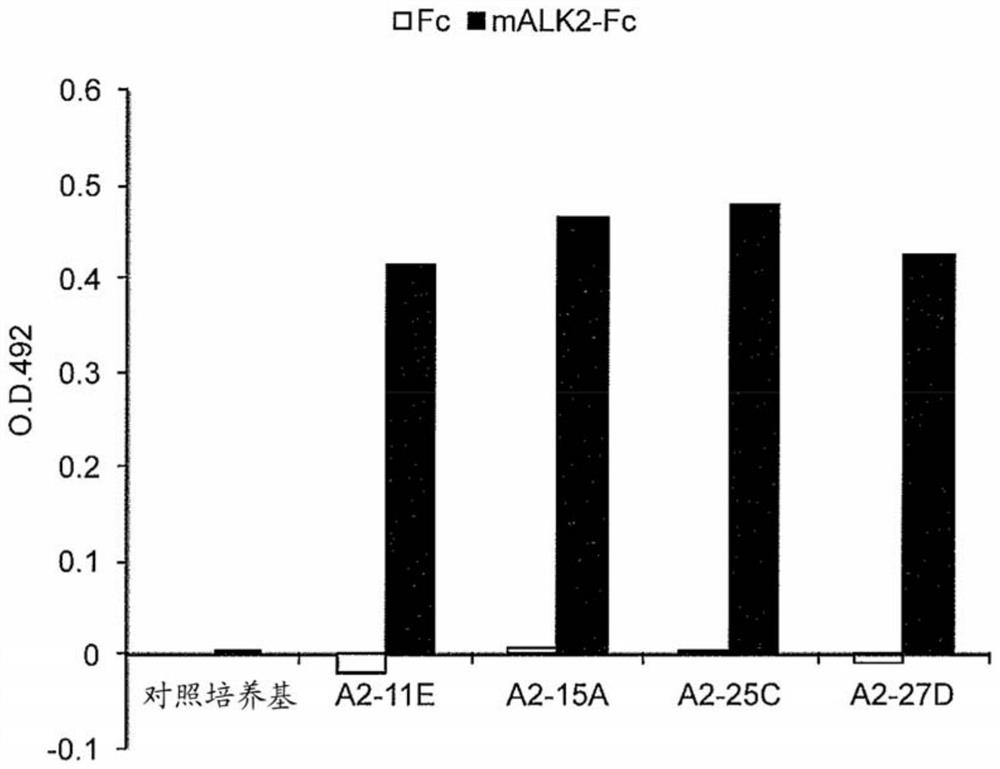
[0500] Preparation of rat anti-ALK2 antibodies (11E2, 15A6, 25C11 and 27D11: hereinafter abbreviated as A2-11E, A2-15A, A2-25C and A2-27D)
[0501] 1) Preparation of -1 antigen
[0502] As mALK2-Fc used as an antigen, mouse ALK2-His&Fc (Cat. #50297-M03H) manufactured by Sino Biological Inc. was used.
[0503] 1)-2 Immunization of animals
[0504] 1 mg / mL of mouse ALK2-His&Fc and TiterMax Gold (TiterMax, USA) were mixed in equal amounts to prepare an emulsion. To two 6-week-old Wister female rats, 100 μg of the antigen was subcutaneously administered together with the adjuvant twice. After 1 to 2 weeks, only 40 μg of the antigen solution was subcutaneously administered, and 3 days later, spleen cells and various lymph nodes were aseptically removed as antibody-forming cells, and used for subsequent cell fusion.
[0505] 1)-3 Cell fusion with myeloma cells
[0506] Adjust the ratio of the above-mentioned antibody-forming cells to the myeloma cell line (P3U1 cells) obtained f...
Embodiment 2
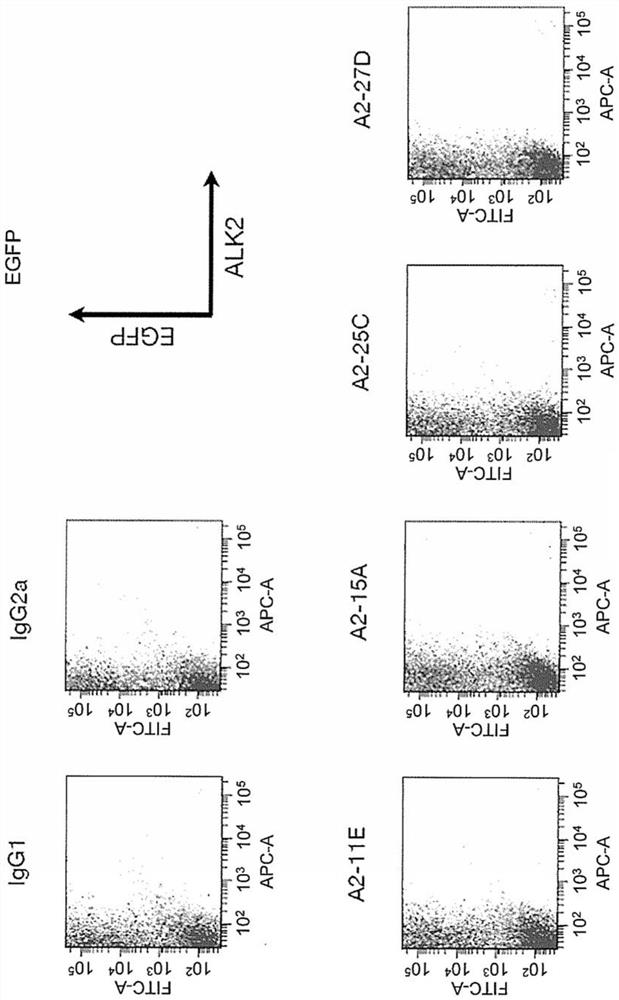
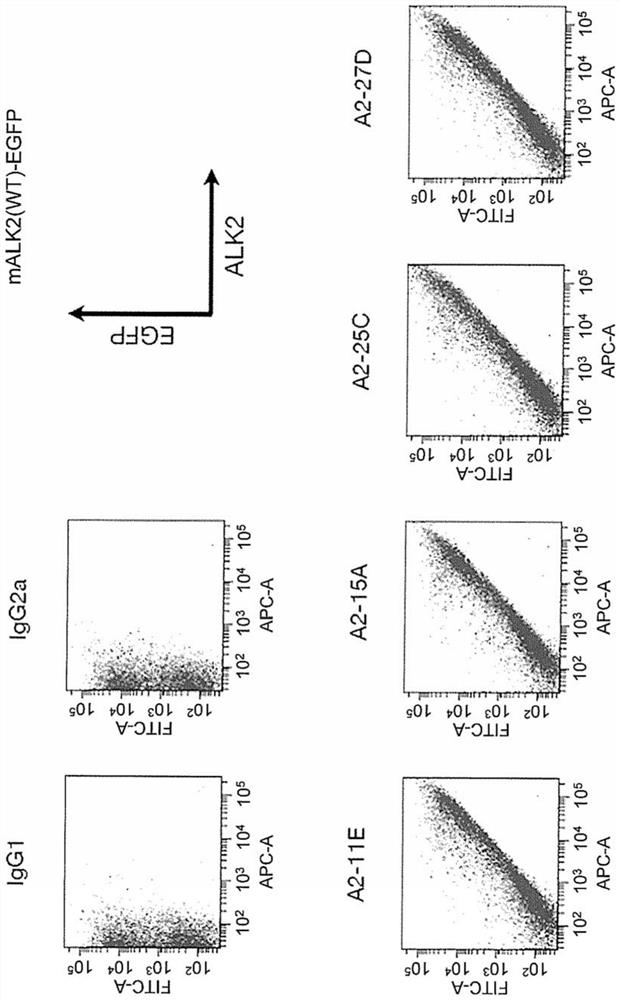
[0513] In vitro (invitro) evaluation of rat anti-ALK2 antibodies (A2-11E, A2-15A, A2-25C and A2-27D)
[0514] 2)-1 Antibody screening by flow cytometry
[0515] 2) Preparation of -1-1 mouse and human ALK2 expressing cells
[0516] HEK293A cells to reach 3 x 10 4 cells / cm 2 Inoculated into 100mm Petri dishes, with DMEM medium containing 15% FBS, at 37°C, 5% CO 2 cultured overnight under conditions. On the next day, pcDEF3 / mouse ALK2(WT)-EGFP, pcDEF3 / human ALK2(WT)-EGFP, pcDEF3 / human ALK2(R206H)-EGFP, and pcDEF3 were introduced into HEK293A cells using Lipofectamine 2000 (manufactured by Invitrogen), respectively, Further overnight incubation. Next day, adjust to 1×10 6 The cells / mL cell suspension was dispensed into 1.5mL microcentrifuge tubes every 100μL, centrifuged at 500g for 5 minutes, and the supernatant was removed. 100 µL of purified IgG diluted to 1 µg / mL was added to the cells, and the cells were left to stand at 4°C for 30 minutes. After the cells were washed...
Embodiment 3
[0536] Determination of the Nucleotide Sequence of the cDNA Encoding the Variable Region of Rat Anti-ALK2 Antibody (A2-11E, A2-15A, A2-25C, A2-27D)
[0537] 3)-1 Determination of the nucleotide sequence of the cDNA encoding the A2-11E variable region
[0538] 3)-1-1 Preparation of total RNA from the hybridoma producing A2-11E
[0539] To amplify the cDNA containing the A2-11E variable region, total RNA was prepared from the A2-11E-producing hybridoma using TRIzol Reagent (Ambion).
[0540] 3) Synthesis of 1-2 cDNA (5'-RACE-Ready cDNA)
[0541] Synthesis of cDNA (5'-RACE-Ready cDNA) was carried out using 1 µg of the total RNA prepared in Example 3)-1-1, and using SMARTer RACE cDNA Amplification Kit (Clontech Corporation).
[0542] 3)-1-3 Amplification and sequence determination of cDNA containing A2-11E heavy chain variable region by 5'-RACE PCR
[0543] As primers for amplifying the cDNA of the variable region of the A2-11E heavy chain gene by PCR, UPM (provided with Univer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com