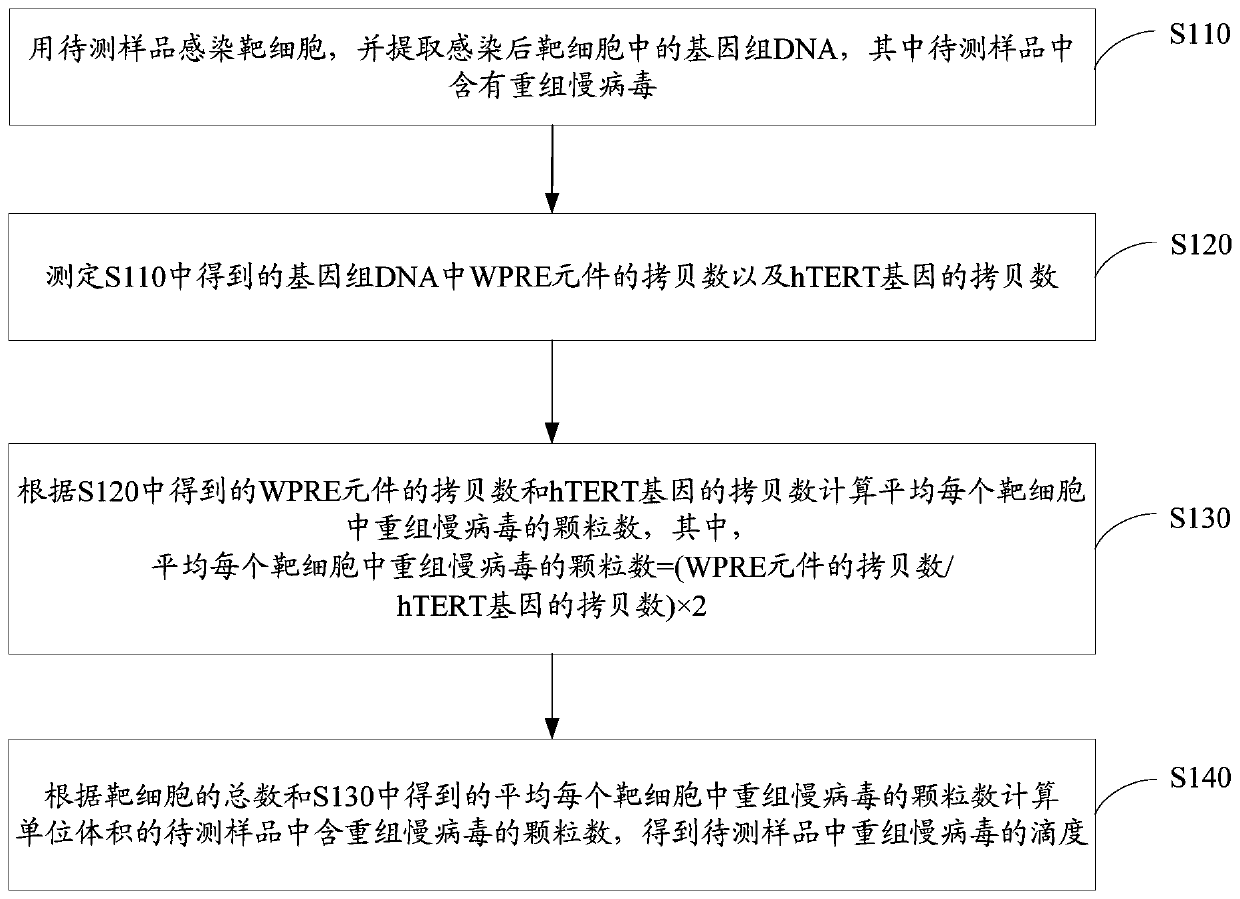
Method for Quantitatively Detecting the Titer of Recombinant Lentivirus
A recombinant lentivirus, quantitative detection technology, applied in microorganism-based methods, biochemical equipment and methods, and microbial assay/inspection, etc., can solve problems such as cumbersome and inconvenient operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The preparation method of the WPRE component standard product is simple, easy and economical, so that a large number of WPRE component standard products can be obtained.
[0076] Of course, in other embodiments, WPRE element standard products can also be obtained by direct base synthesis.
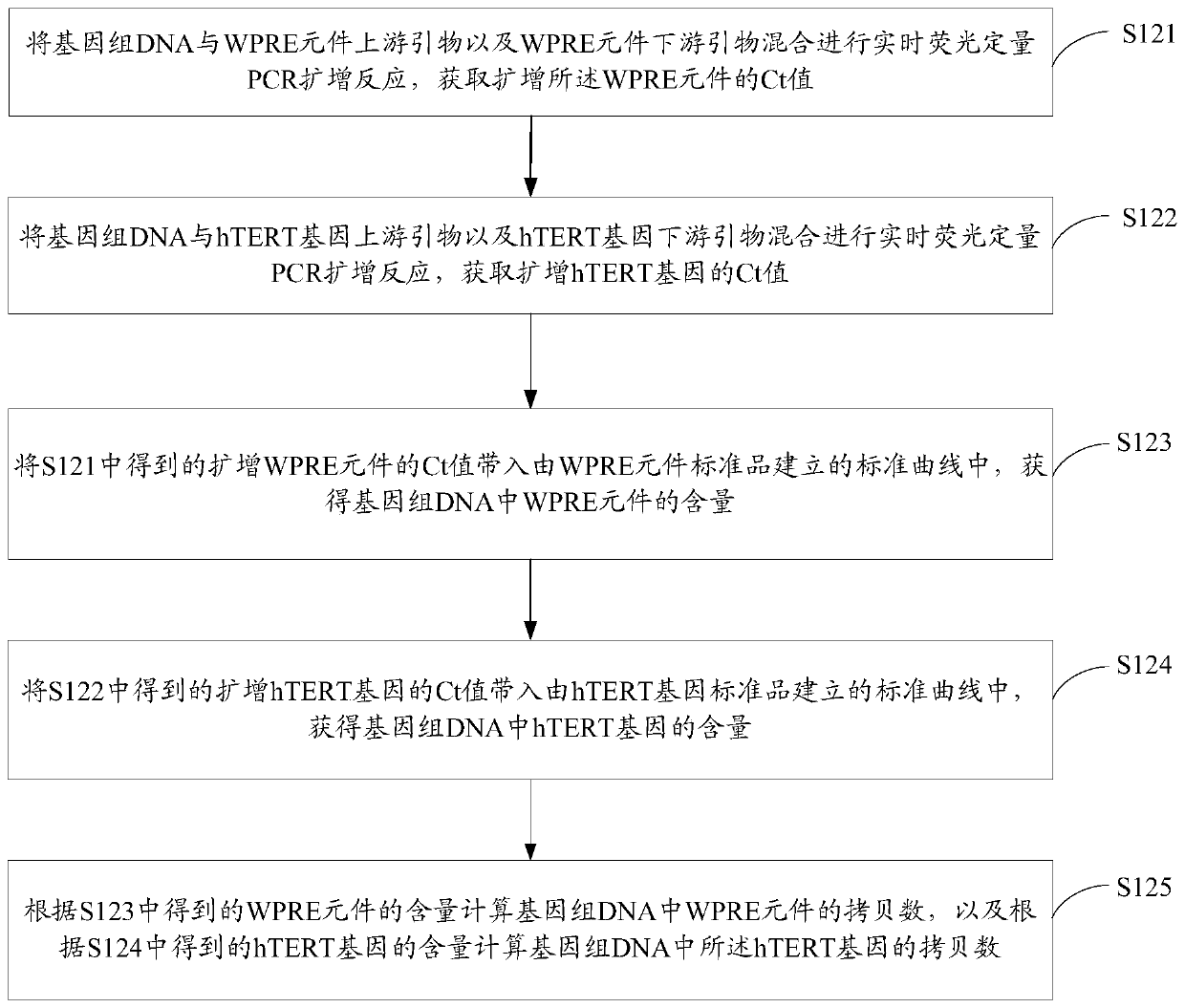
[0077] S124. Put the Ct value of the amplified hTERT gene obtained in S122 into the standard curve established by the hTERT gene standard to obtain the content of the hTERT gene in the genomic DNA.
[0078] The standard curve established by the hTERT gene standard product has a corresponding relationship between the Ct value and the hTERT gene content. According to the Ct value of the amplified hTERT gene, the content of the hTERT gene in the genomic DNA can be quantitatively calculated, that is, the content of the hTERT gene.
[0079] In one embodiment, the standard curve established by the hTERT gene standard product is obtained by the following operations: the hTERT gene standard ...
Embodiment 1
[0105] Preparation of WPRE element standard and hTERT gene standard
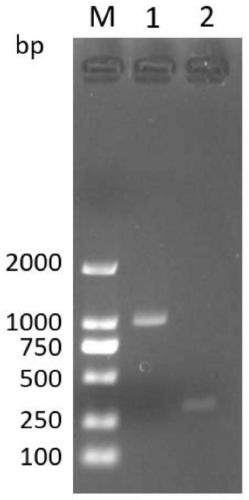
[0106] Referring to the sequence of the WPRE element template synthesized with the accession number NC_004107.1 in the GenBank database, the upstream primer of the WPRE element template shown in SEQ ID No.5 and the upstream primer of the WPRE element template shown in SEQ ID No.6 were added according to the system recommended by the PrimeSTAR HS (Premix) kit. Show the downstream primers of the WPRE element template, and carry out PCR amplification reaction. The reaction conditions are 98°C for 10s, 1 cycle; 98°C for 10s, 55°C for 10s, 72°C for 60s, 30 cycles; 72°C for 5min, 1 cycle.
[0107] Referring to the sequence of the hTERT gene template synthesized with the accession number NG_009265.1 in the GenBank database, the upstream primer of the WPRE element template shown in SEQ ID No.7 and the upstream primer of the WPRE element template shown in SEQ ID No.8 were added according to the system recommended by t...
Embodiment 2
[0111] Establish a standard curve for the WPRE element standard and a standard curve for the hTERT gene standard
[0112] Take 2 μL of the WPRE component standard with a concentration of 0.5ng / μL (the quality is 1ng), and perform a gradient dilution with a factor of 8, dilute 7 gradients (the minimum content is diluted 2097152 times), and obtain a total of 8 WPRE component standards . Using these 8-content WPRE element standard products as templates, respectively add the WPRE element upstream primer shown in SEQ ID NO: 1 and the WPRE element downstream primer shown in SEQ ID NO: 2 to perform real-time fluorescent quantitative PCR amplification reaction. Reaction conditions It is: 95°C for 30 seconds, enter the cycle; 95°C for 5 seconds, 60°C for 30 seconds, end-point plate reading, a total of 40 cycles. get as Figure 4 The amplification curve shown and as Figure 5 Melting curve shown. according to Figure 4 Amplification curves are shown to obtain Ct values for each l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com