Improved T cell therapy
A cellular and therapeutic technology, applied in genetically modified cells, gene therapy, animal cells, etc., can solve the problem of not being able to fundamentally promote the immune memory of CAR-T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Embodiment 1, the production and characterization of Raji-B-NDG mouse model
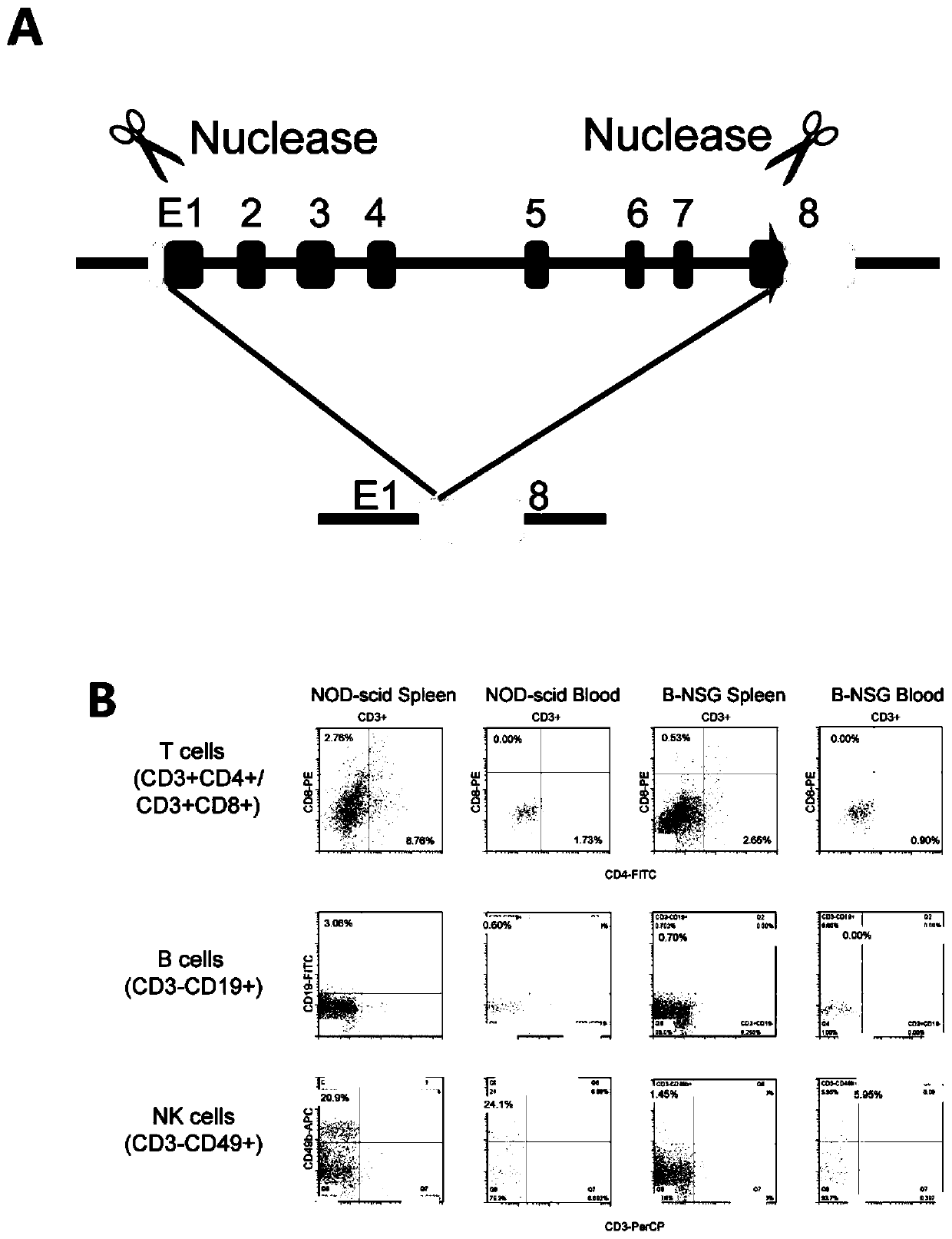
[0114] Nonobese diabetic severe combined immunodeficiency (NOD-scid) mice with null Il2rg lack mature T, B, and natural killer (NK) cells and lack cytokine signaling, leading to better engraftment of multiple cancer types . In this embodiment, the Il2rg gene was deleted from NOD-scid mice using CRISPR / Cas9 genome editing technology, thereby preparing B-NDG mice ( figure 1 A). B-NDG mice were observed to be viable, fertile and did not display any obvious physical abnormalities. Compared with NOD-scid mice, FACS analysis showed that the spleen and peripheral blood of B-NDG mice had lower numbers of CD19+ B cells, CD4+ and CD8+ T cells ( figure 1 B). NOD-scid mice had a high percentage of CD49b+ NK cells (spleen 20.90%, peripheral blood 24.10%), while B-NDG mice had no functional NK cells (spleen 1.45%, peripheral blood 5.95%). Therefore, B-NDG mice generated by genome editing are phenotypic...
Embodiment 2
[0117] Example 2, In vitro and in vivo efficiency of CD19-targeted CAR-T cells
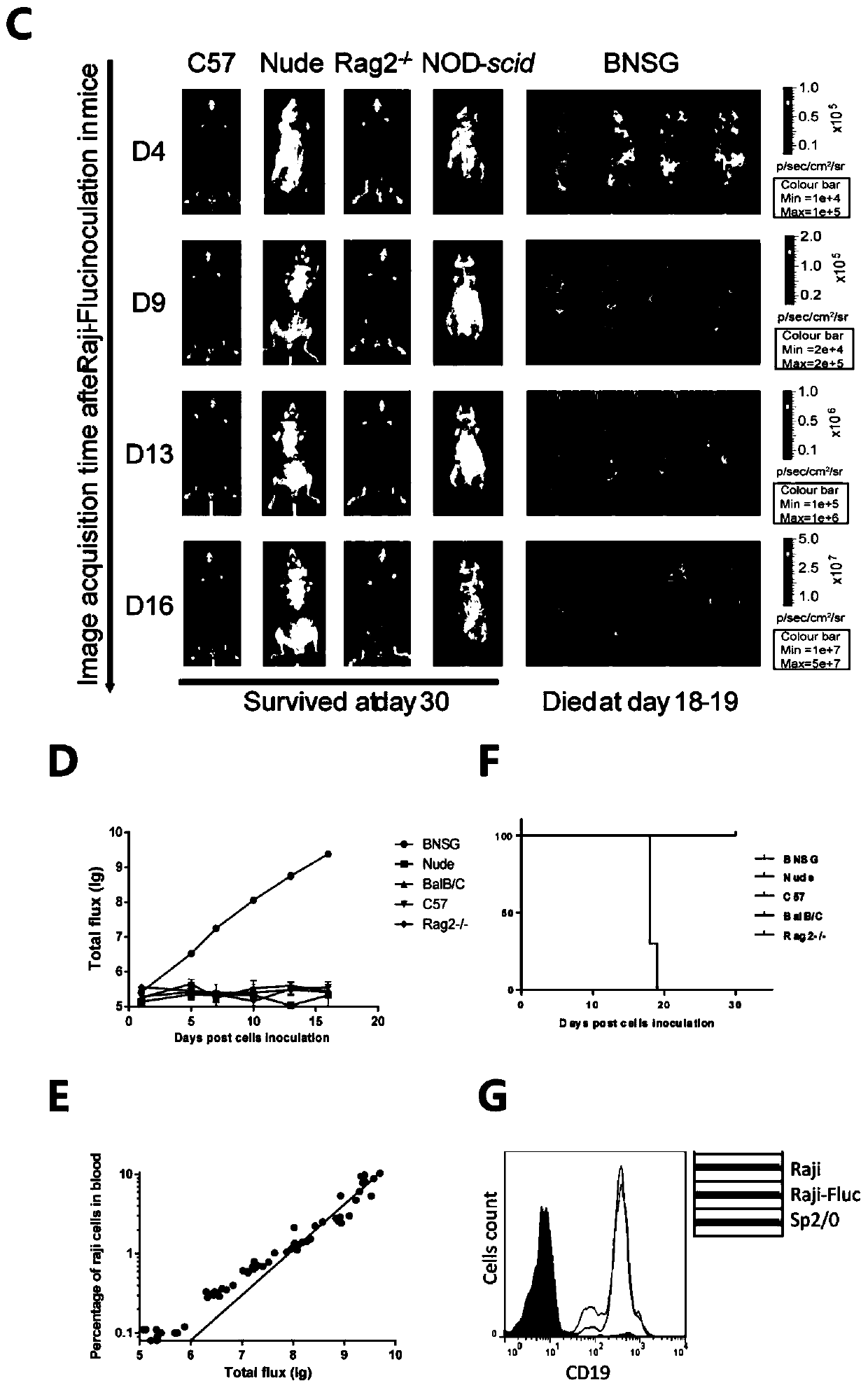
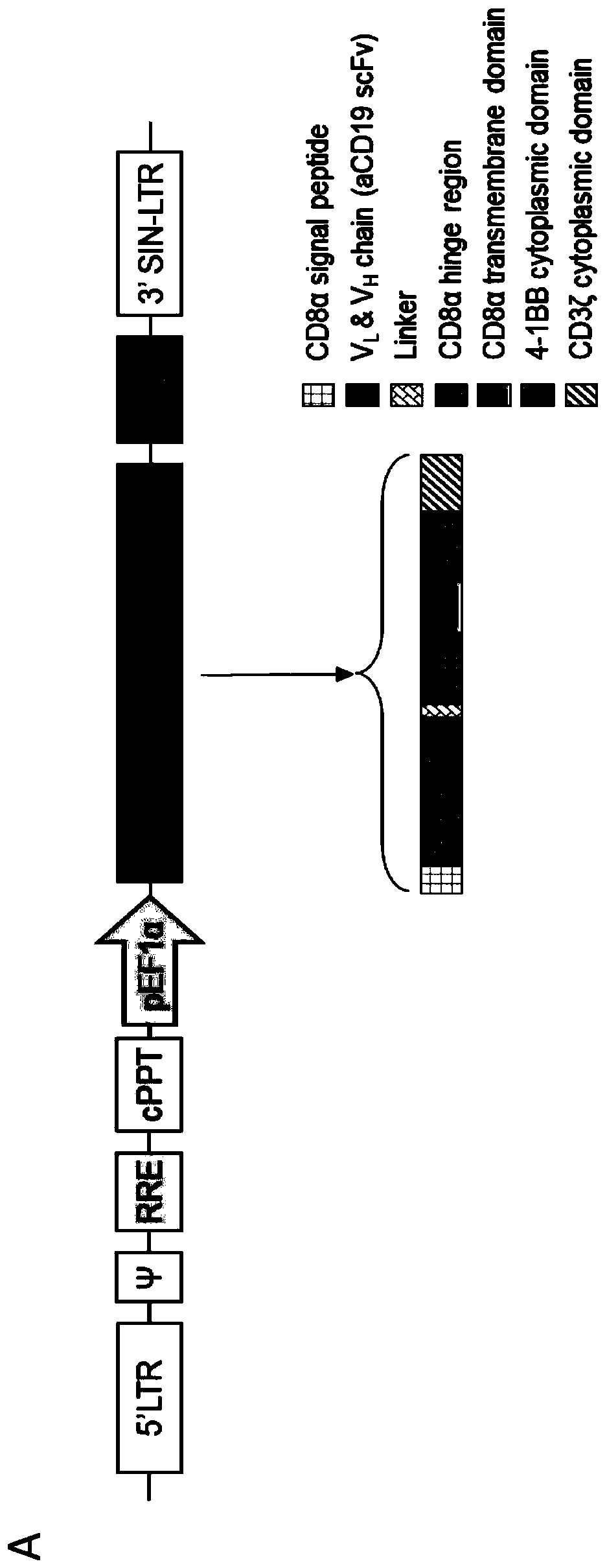
[0118] After T cell activation, PBMCs isolated from volunteers were treated with second-generation CAR ( figure 2 A) Perform transduction. The results showed that the proportion of CD19CAR-T cells increased with the prolongation of culture time, reaching 20% at D12, and the total number of cells was greater than 1×10 at D10 after infection. 8 ( figure 2 B). When co-cultured with CD19+ Raji cells in vitro, CD19CAR-T cells cultured for 12 days showed more than 50% killing effect on Raji cells when the effector cell / target cell ratio was about 3:1. In order to further verify the anti-tumor effect of CD19CAR-T cells in vivo, B-NDG mice were injected with 5×10 5 Raji-Fluc cells, followed by implantation of 2 × 10 7 CD19CAR-T cells. After four days of CD19CAR-T treatment (D9), the bioluminescent signal of these treated mice was close to that of uninoculated mice ( image 3 A), showing that CD...
Embodiment 3
[0119] Embodiment 3, the recurrence source of Raji tumor
[0120] Although CAR-T cells were effective, B-NDG mice eventually died of tumors. To find the source of the Raji cell relapse, all major organs of the mice were tested for bioluminescence. The results showed that Raji cells were significantly cleared by CD19CAR-T cells in spleen, heart and skin. For example, in the spleen, bioluminescence was reduced by 1000-fold after CAR-T cell treatment ( image 3 A). However, CD19CAR-T was not as effective in other organs, especially in the liver and brain ( image 3 A). Residual Raji cells hidden in these organs that cannot be effectively cleared by CAR-T may be the cause of tumor recurrence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com