Method of treating idiopathic thrombocytopenia purpura (ITP) with romiplostim
A technology of romiprostim and platelets, which is applied in the field of treating idiopathic thrombocytopenic purpura (ITP) with romiprostim, and can solve the problems of compliance, large cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
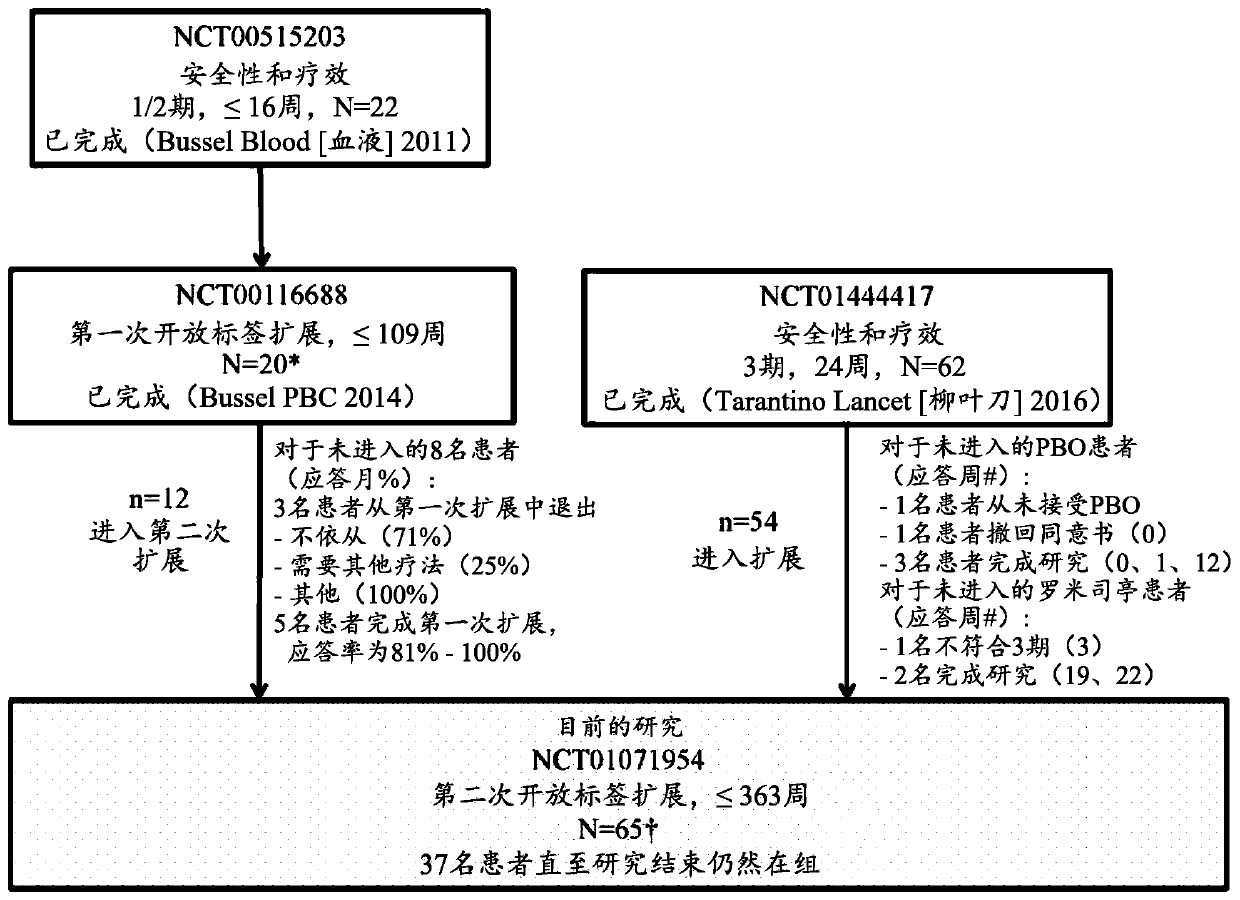
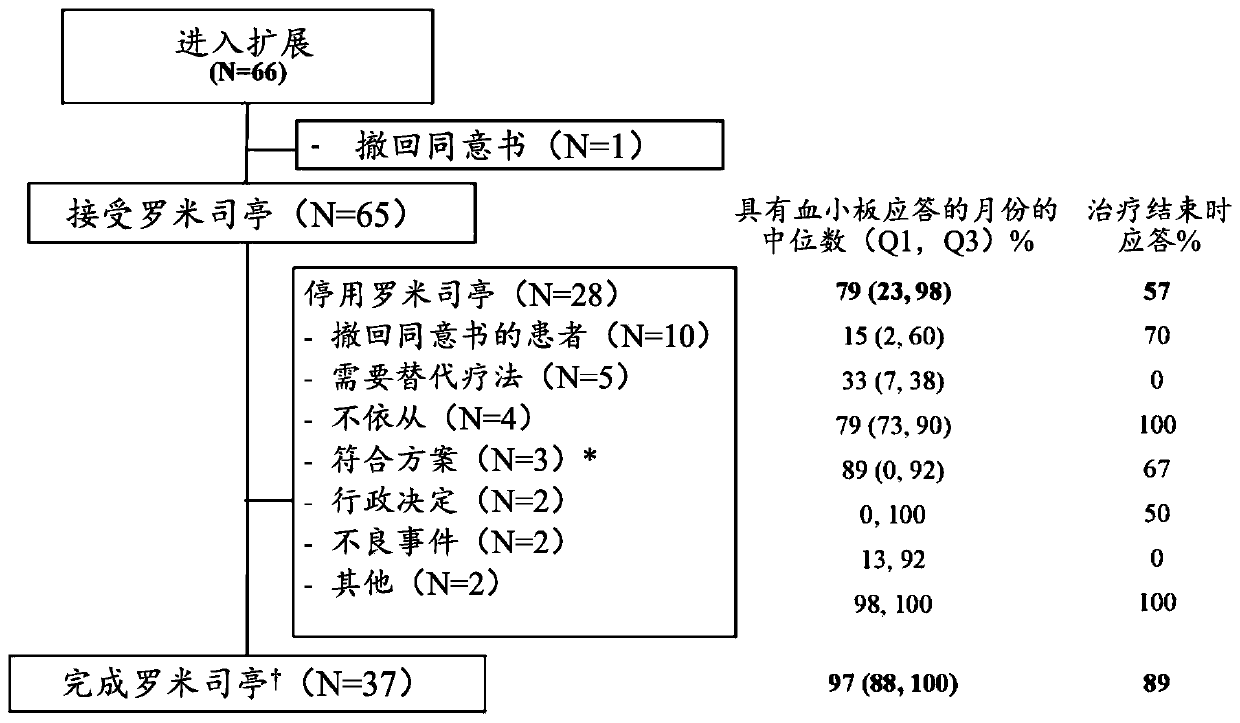
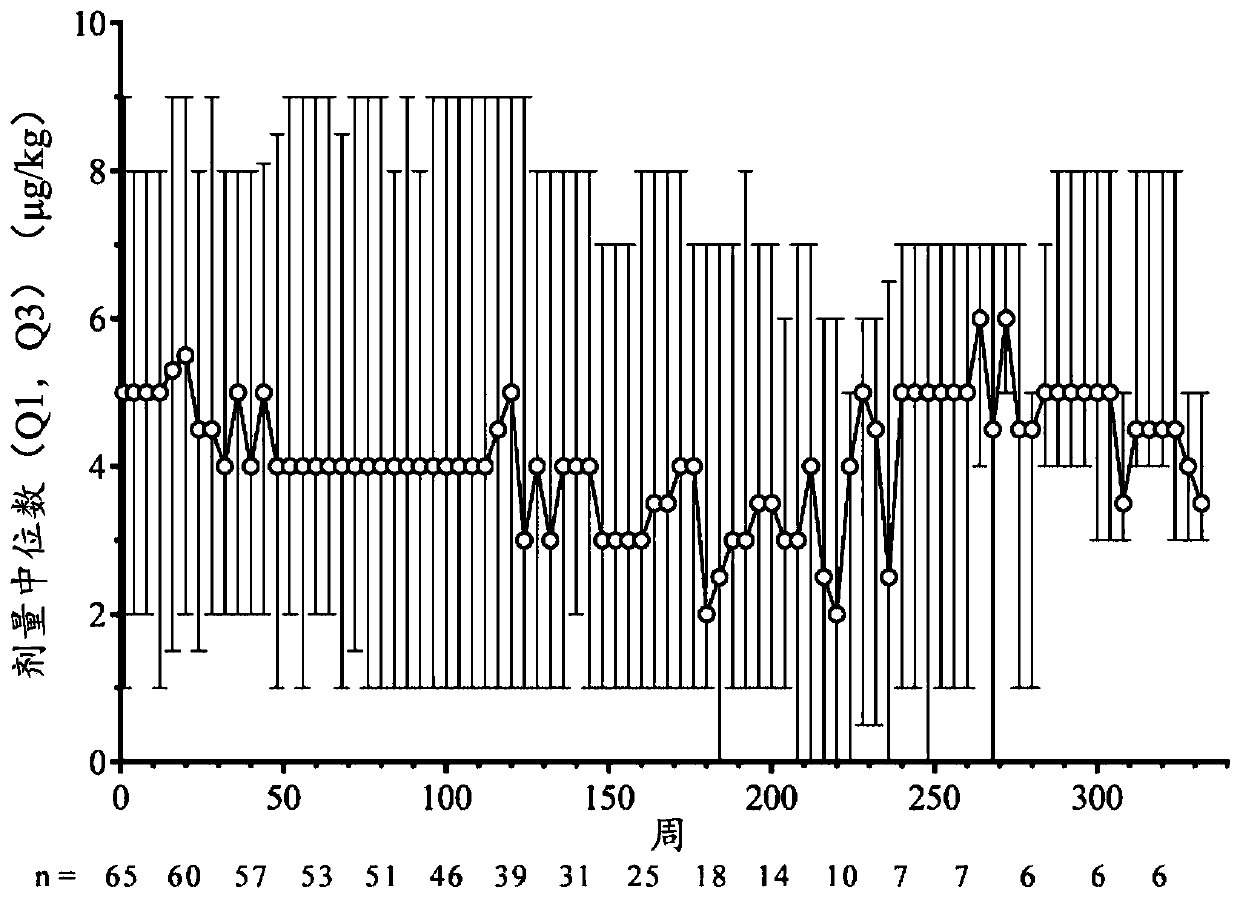
[0023] TPO receptor agonists are not an ideal treatment option for ITP because not every patient responds, and those who do respond are not always able to maintain their response. The present invention is based on the largest study to date conducted in children using TPO receptor agonists, with over 182 patient-years of exposure, or 2.8 years of exposure per patient for 65 patients. Importantly, approximately 1 / 4 of patients were able to discontinue ITP therapy and still maintain a hemostatic platelet count. The data described in this manuscript are extensive both in terms of number of patients (n=65) and duration of treatment (up to 7 years) and show that romigrastim has efficacy and demonstrates a safety profile similar to that seen in adults characteristic.
[0024] The aim of the study was to describe the safety and efficacy of long-term use of romigrastim in children with ITP, with the incidence of adverse events as the primary endpoint. Secondary endpoints included ass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com