Maintenance therapy of a parp inhibitor in treating gastric cancer
A technology for inhibitors and gastric cancer, applied to medical preparations containing active ingredients, drug combinations, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
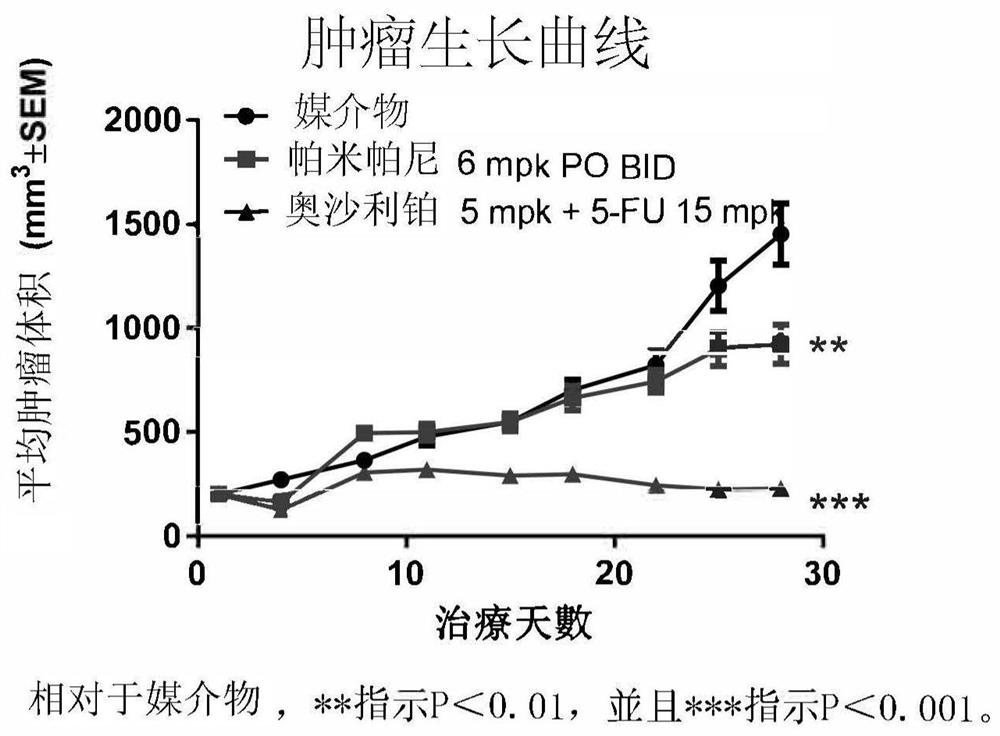
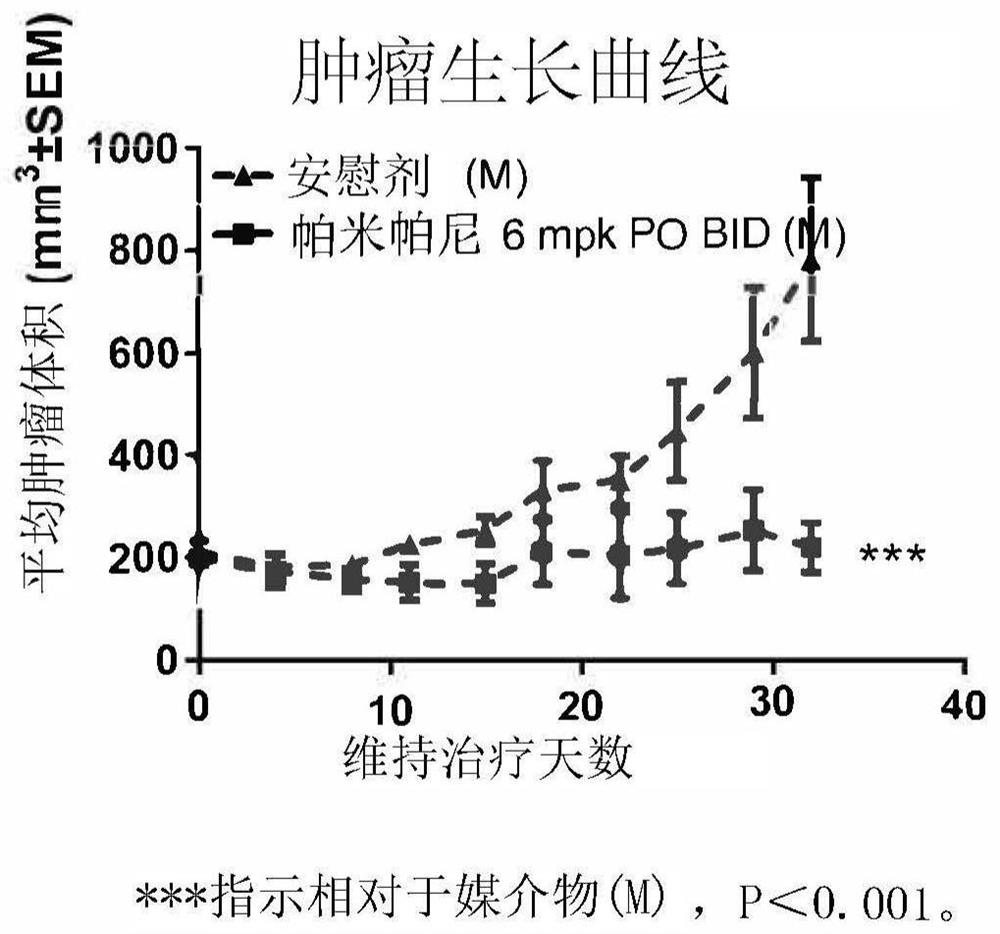
[0035] Example A: Preclinical Studies
[0036] A Study of Pamiparib Versus Placebo as Maintenance Therapy in a BBGA087 Patient-Derived Xenograft Model of Gastric Cancer Responsive to Oxaliplatin
[0037] method
[0038] BBGA087 patient-derived gastric cancer tissue fragments (3 × 3 × 3 mm3) were implanted subcutaneously in the right flank of female nod-scid mice. After inoculation, tumor volume and body weight were measured twice a week and calculated with the formula: V=0.5×(a×b2), where a and b are the long and short diameters of the tumor, respectively. When the average tumor size reached 200mm3, the animals were randomly divided into 3 groups. Animals were treated with vehicle (0.5% methylcellulose, 0.5% MC), pamiparib, oxaliplatin+5-fluorouracil (5-FU), respectively. BGB290 was given by oral gavage (p.o.) at 6 mg / kg twice a day (BID); oxaliplatin was given by intraperitoneal (i.p.) injection once a week (QW) for three weeks; and 5-FU was administered by intraperiton...
Embodiment B
[0053] Example B: Clinical Study
[0054] method
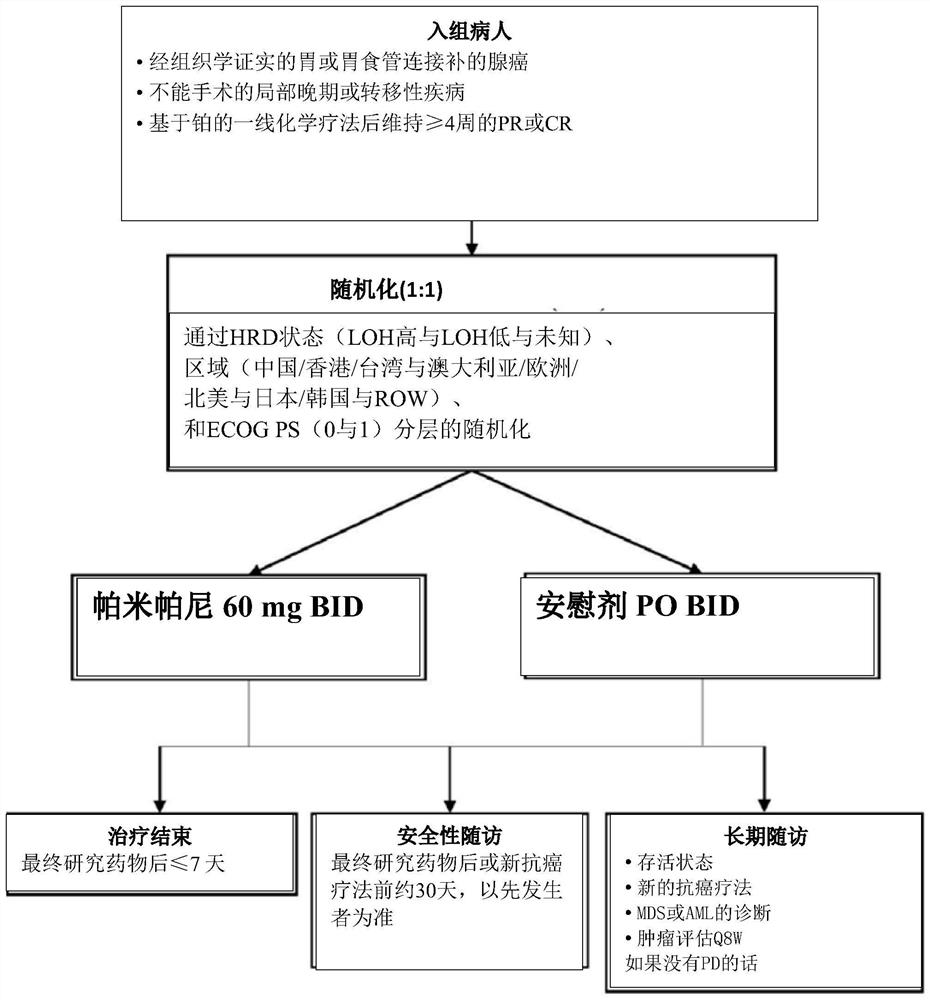
[0055] Overall Design and Research Objectives
[0056] A phase 3, double-blind, placebo-controlled, randomized, multicenter study was designed to compare the efficacy, safety of pamiparib versus placebo as maintenance therapy in patients with advanced gastric cancer responding to platinum-based first-line chemotherapy and tolerance ( image 3 ).
[0057] The primary objective was to evaluate the maintenance efficacy of pamiparib compared to placebo in terms of progression-free survival (PFS) as assessed by a blinded independent review committee (BIRC).
[0058] Secondary objectives would include pamiparib versus placebo in other efficacy assessments (overall survival [OS]; investigator-assessed PFS; PFS at 2 years [PFS2]; time to second follow-up therapy [TSST ]; and Objective Response Rate [ORR], Duration of Response [DoR], and Time to Response, all assessed by the investigator) and a comparison of safety and tolerability...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com