A method for inducing myogenic differentiation of adipose-derived mesenchymal stem cells
A technology derived from stem cells and adipose tissue, applied in the field of myogenic differentiation of stem cells, can solve the problems that the role of myoblast differentiation has not been reported, and achieve the goal of improving myogenic differentiation, increasing MyoD expression, and promoting myogenic differentiation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A eight-week large male New Zealand White Rabbit (Jiangsu University Animal Experiment Center) with a weight of 2.0kg (Jiangsu University Animal Experiment Center), raising and feeding rabbits under single cage feeding conditions. Control the temperature of the house (25 ° C), humidity (40-60%) and illumination (12h, bright and dark cycle). Observe the animal for a week before surgery to confirm that they are healthy.
[0045] In order to obtain ADSC, the animals were euthanized into the anesthesia chamber by passively inhaled the isoflurane until an excess occurred. The death of the rabbit is evaluated by monitoring the heart-breathing stop. Cut the hair from the abdomen area, cut a slit to expose the peritoneum, and remove the groin fat. The adipose tissue is then placed in a cellular chamber. The adipose tissue was washed 3 times with phosphate buffered saline (PBS) to remove red blood cells. The collected adipose tissue was cut into a small piece and transferred to a 20...
Embodiment 2
[0046] Example 2 Evaluation of Separation of Cells
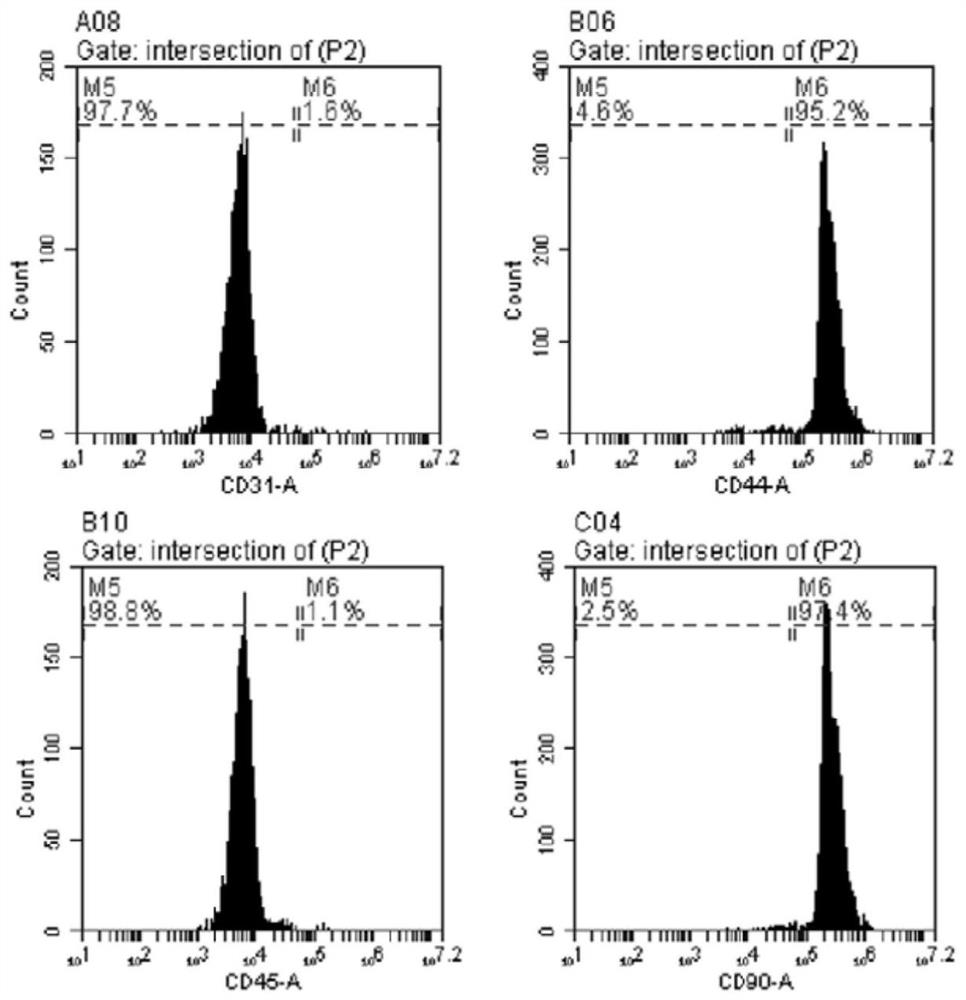
[0047] There is no specific antigen in the MSC. Therefore, there is a need to simultaneously analyze a variety of surface antigen and cell morphology to determine the characteristics of mesenchymal cells. The ADSC surface marker is checked by flow cytometry. The third generation adhesive cells were treated with 0.25% trypsin (GIBCO, US) and washed twice with PBS. Cells were incubated with rabbit anti-CD31, anti-CD44, anti-CD45 and anti-CD90 antibodies (US Invitrogen and US GIBCO) were incubated at 4 ° C overnight. Unbound antibodies were removed by washing with PBS. After washing, cells were incubated with Cy3 labeled anti-goat / anti-rabbit secondary antibody incubation for 45 minutes at room temperature, and then resuspended in PBS for FACS analysis. Analysis of at least 1 × 10 per sample with flow cytometry (BD FACSVERSE, USA) 6 Cells.
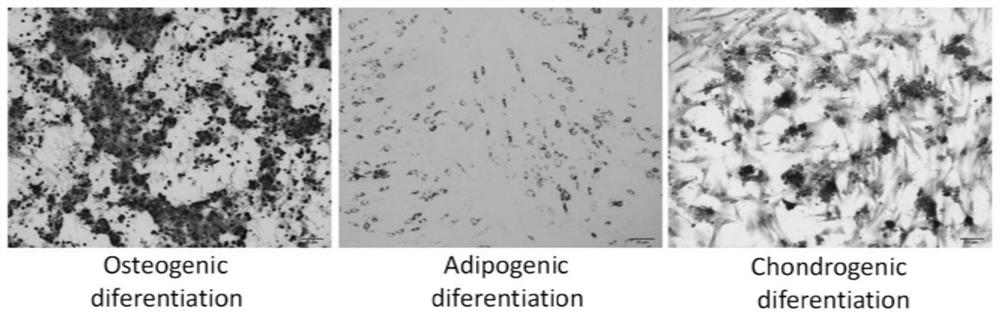
[0048] In this study, the cells separated from adipose tissue have the following characte...
Embodiment 3
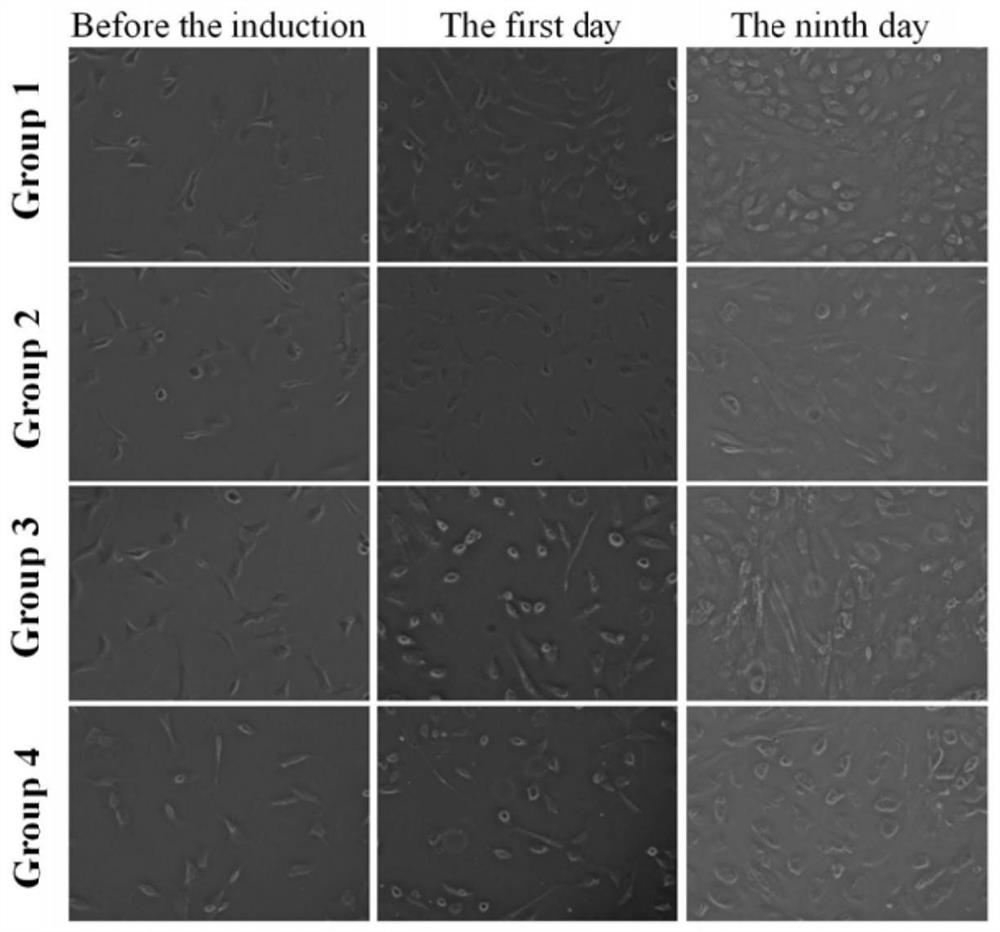
[0051] Third generation cells are digested with mixtures of trypsin and EDTA, 4 Single-cell suspensions of cells / mL then inoculated into a 6-well plate. The cells were divided into 4 groups. The first group is control group; the second group of inducers were treated with induced agent; the third group of inducers H 2 Treatment; 4th group of inducers 5-AZA + H 2 handle. The second group and the fourth group were induced with 10 μmol / l 5-Aza (Sigma, US) for 24 h, and was rinsed with D-HANKS equilibrium salt solution; then the medium was replaced with a high glucose DMEM containing 15% FBS. Group 1 and Group 2 are at 37 ° C and 5% Co in a conventional incubator. 2 Incubate together, while the third group and 4th group were in a hydrogen-containing incubator at 37 ° C, 25% h 2 Incubate; the oxygen concentration is 21%, the humidity is maintained in more than 90%, and the concentration is 5% concentration in the culture environment. 2 N-Ni NP 9% 2 . The medium was replaced with fre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com