Method for inducing myogenic differentiation of adipose-derived mesenchymal stem cells
A technology derived from stem cells and adipose tissue, applied in the field of myogenic differentiation of stem cells, can solve the problems that the role of myoblast differentiation has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] An eight-week-old male New Zealand white rabbit (Jiangsu University Animal Experiment Center) with a body weight of 2.0 kg was used to raise and feed the rabbits under single-cage rearing conditions. The temperature (25° C.), humidity (40-60%) and light (12h, light-dark cycle) of the house were controlled. Animals were observed for one week prior to surgery to confirm that they were healthy and disease-free.
[0045] To obtain ADSCs, animals were euthanized in an anesthesia room by passive inhalation of isoflurane until overdose occurred. Rabbits were assessed for death by monitoring for cardio-respiratory arrest. Hair was clipped from the abdominal region, an incision was made to expose the peritoneum, and inguinal fat was removed. Then, place the adipose tissue in a laminar flow chamber. Adipose tissue was washed 3 times with phosphate-buffered saline (PBS) to remove red blood cells. The collected adipose tissue was cut into small pieces and transferred to a 20 mL...
Embodiment 2
[0046] Evaluation of Example 2 Isolated Cells
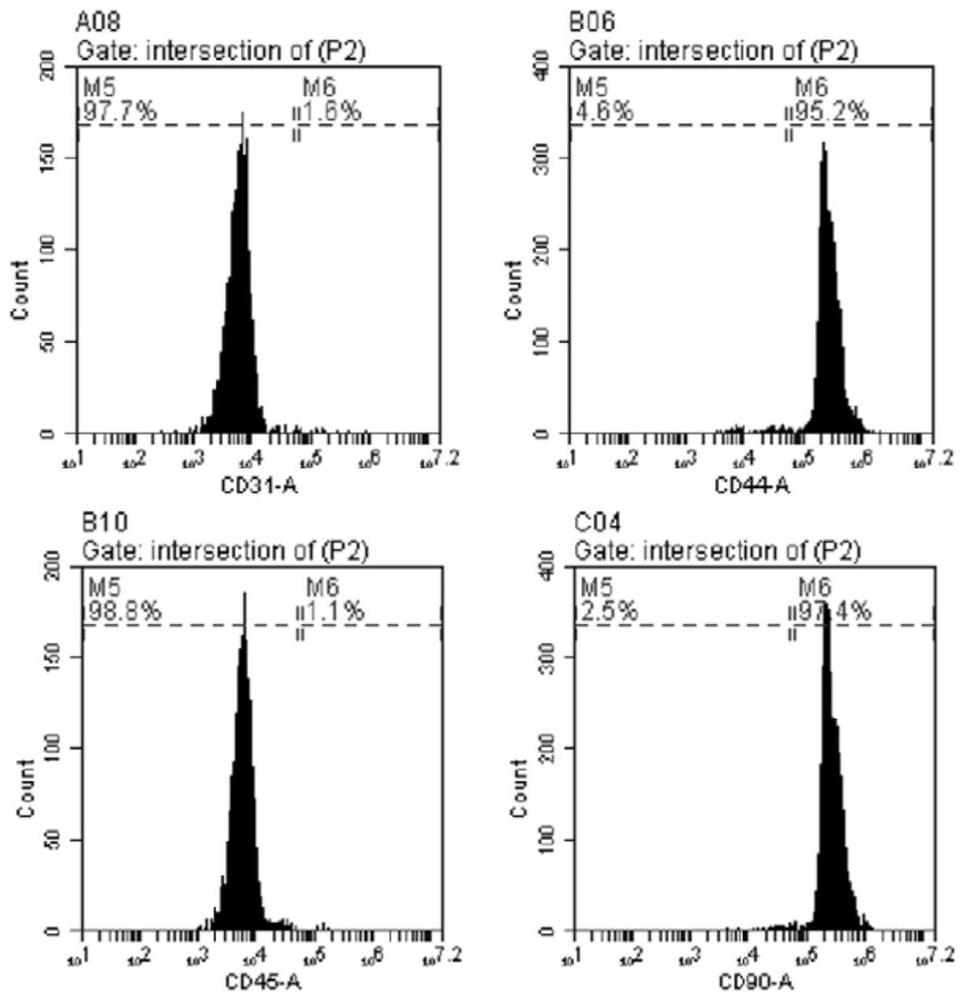
[0047] There are no specific antigens in MSCs. Therefore, simultaneous analysis of multiple surface antigens and cell morphology is required to characterize mesenchymal cells. ADSC surface markers were examined by flow cytometry. Passage 3 adherent cells were treated with 0.25% trypsin (Gibco, USA) and washed twice with PBS. Cells were incubated overnight at 4°C with rabbit anti-CD31, anti-CD44, anti-CD45 and anti-CD90 antibodies (Invitrogen, USA and Gibco, USA). Unbound antibody was removed by washing three times with PBS. After washing, cells were incubated with Cy3-labeled anti-goat / anti-rabbit secondary antibody for 45 min at room temperature in the dark, then resuspended in PBS for FACS analysis. Each sample was analyzed with a flow cytometer (B D FACSVerse, USA) at least 1 × 10 6 cells.
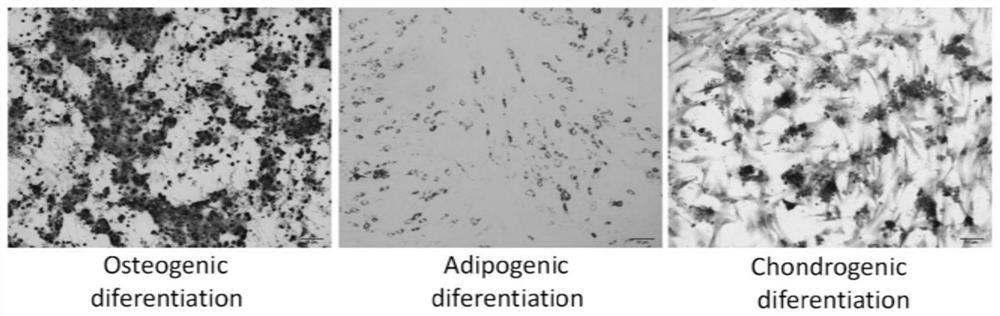
[0048] In this study, cells isolated from adipose tissue were characterized by (1) spindle-shaped adherent growth and colony format...
Embodiment 3
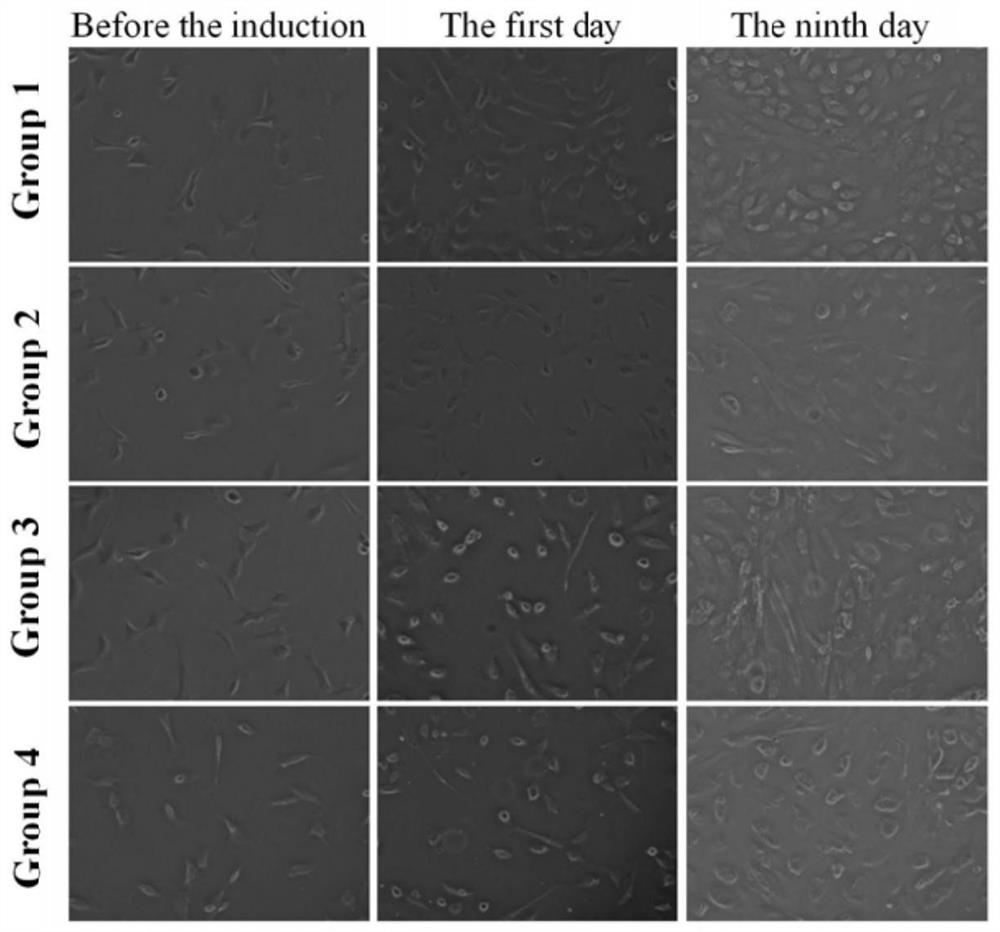
[0051] The third passage cells were digested with a mixture of trypsin and EDTA to make 10 4 cells / mL of single-cell suspension, and then seeded into 6-well plates. Cells were divided into 4 groups. Group 1 was the control group; Group 2 was treated with inducer 5-Aza; Group 3 was treated with inducer 5-Aza+H 2 treatment; group 4 with inducer H 2 deal with. Cells in groups 2 and 4 were induced with 10 μmol / L 5-Aza (Sigma, USA) for 24 hours, and washed with D-Hanks balanced salt solution; then, the medium was replaced with high glucose DMEM containing 15% FBS. Groups 1 and 2 were incubated in a conventional incubator at 37 °C and 5% CO 2 Incubate together while groups 3 and 4 at 37 °C in a hydrogen incubator, 25% H 2 Incubation; the oxygen concentration is 21%, the humidity is kept above 90%, and the culture environment contains a concentration of 5% CO 2 , and a concentration of 49% N 2 . The medium was replaced with fresh DMEM and FBS every 3 days until the test was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com