Polyvalent derivatives of emoxypine
A technology of amoxipine and its derivatives, applied in the field of treatment of synuclein disease, can solve problems such as undiscovered disease improvement treatment methods, movement disorders and mental symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
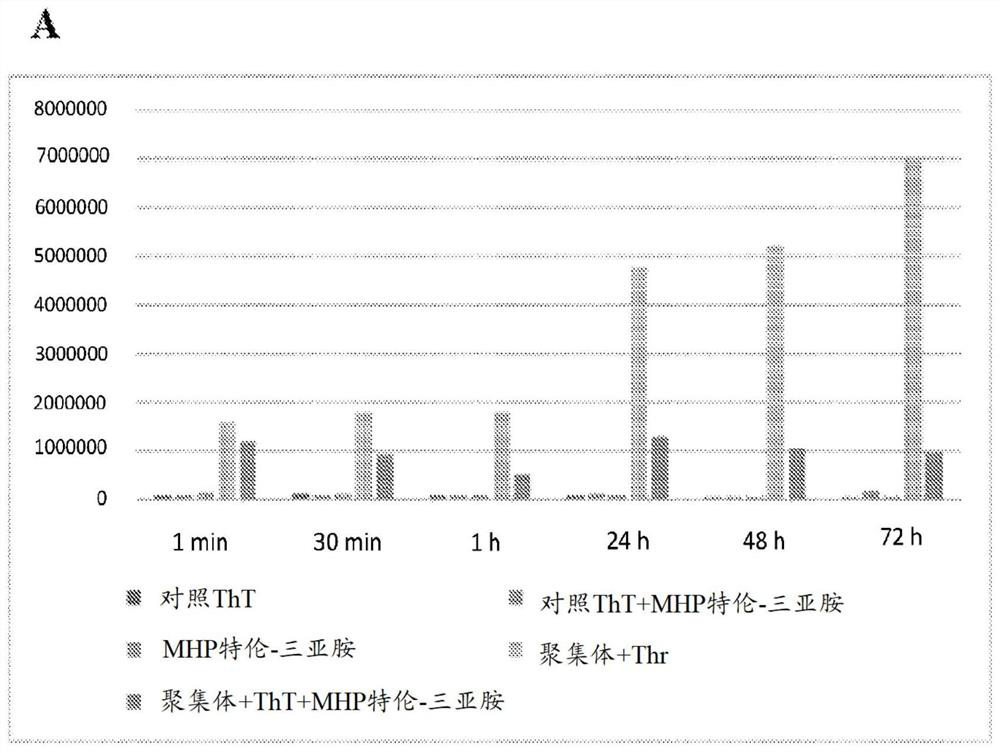
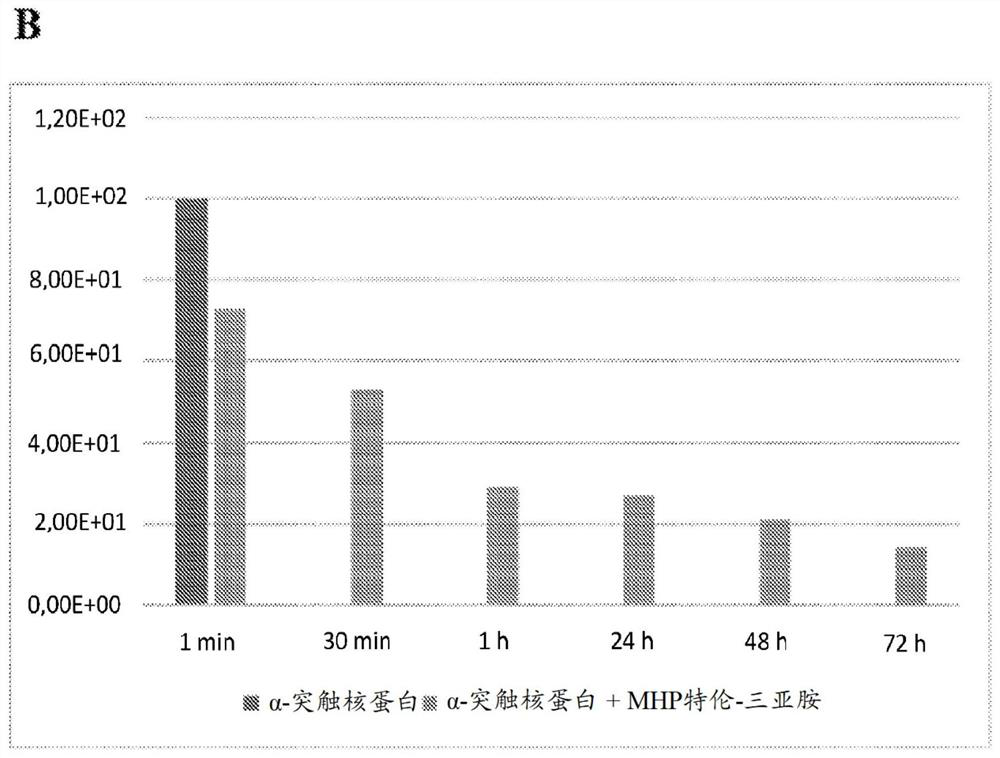
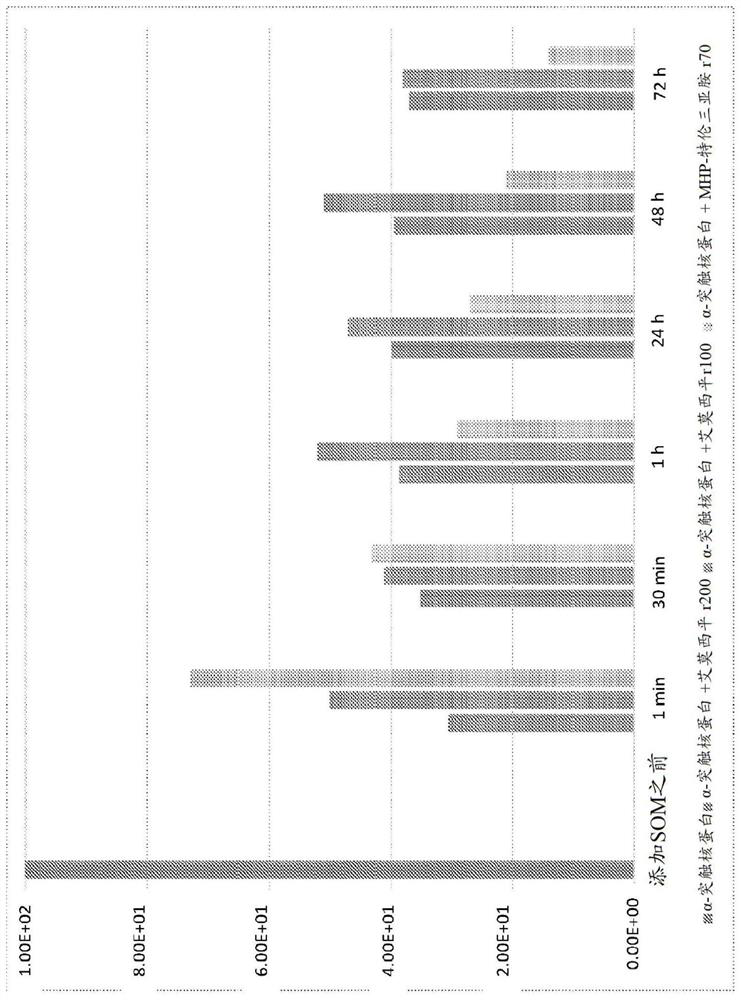
[0217] Example 1: Experiments related to the depolymerization of α-synuclein using tetraiminoemoxipine
[0218] Depolymerization of α-synuclein by tetraiminoamoxipine.
[0219] An assay measuring the activity of tetraimino-amoxipine for solubilizing α-synuclein aggregates was performed.
Embodiment 2
[0220] Example 2: Synthesis of Tetraimino Amoxazepine Derivatives and Polyamido Amoxazepine Derivatives
[0221] The polyamido amoxazepine derivatives and the tetraimino amoxazepine derivatives of the present invention are synthesized respectively by the following formation methods:
[0222]
Embodiment 3
[0223] Example 3: Synthesis of Triimino Amoxepine Derivatives
[0224] Triimino-amoxipine (also known as MHP-trentriimine) derivatives of the present invention are synthesized via the following formation scheme:
[0225]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com