Methods for treating muscular dystrophy with casimersen
A technology for muscular dystrophy and Duchenne muscular nutrition, applied in gene therapy, pharmaceutical formulations, muscular system diseases, etc., can solve the problem of limited pharmacological options for DMD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Example 1: ESSENCE
[0183] ClinicalTrials.gov Identifier: NCT02500381
[0184] The primary objective of this study was to evaluate the efficacy of Coximosen (SRP-4045) and Golodirsen (SRP-4053) compared to placebo with the ability to skip exon 45 and exon 53, respectively. Efficacy in Duchenne muscular dystrophy (DMD) patients with out-of-frame deletion mutations.
[0185] Research Type: Intervention
[0186] Study Design: Allocation: Random
[0187] Intervention Mode: Parallel Assignment
[0188] Shielding: Quadruple (Participant, Care Provider, Investigator, Outcome Assessor)
[0189] Primary Purpose: Healing
[0190] Title: A double-blind, placebo-controlled, multicenter study with an open-label extension to evaluate the efficacy and safety of SRP-4045 and SRP-4053 in patients with Duchenne muscular dystrophy
[0191] Materials and methods
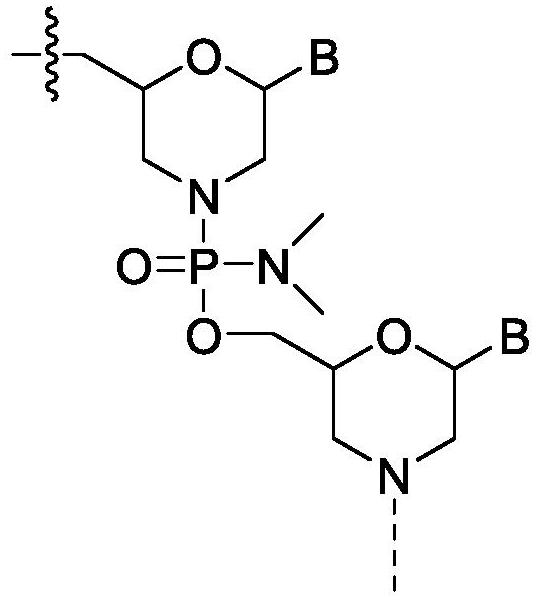
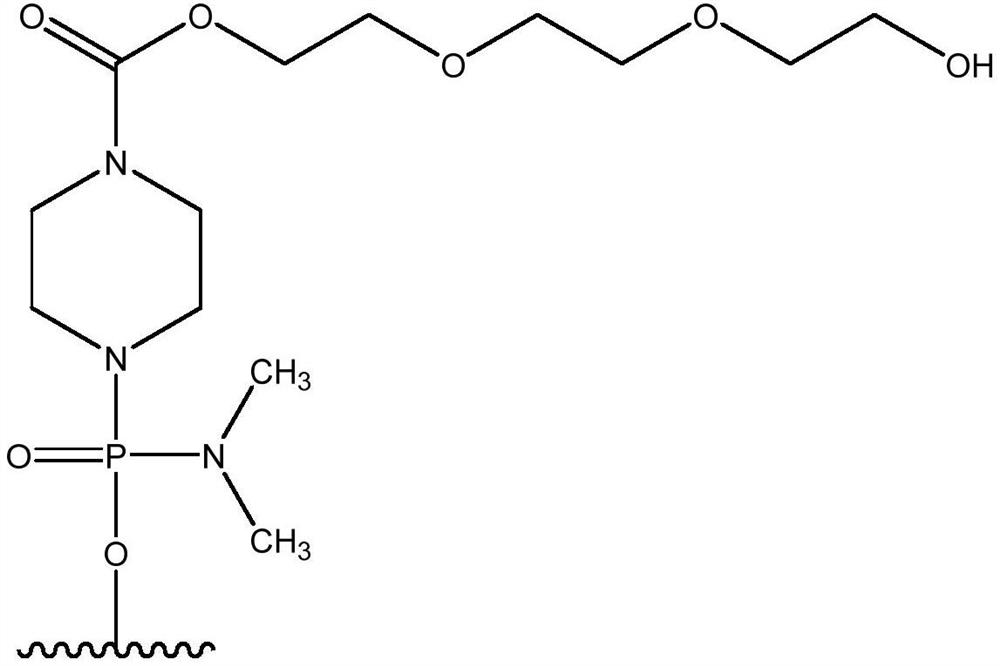
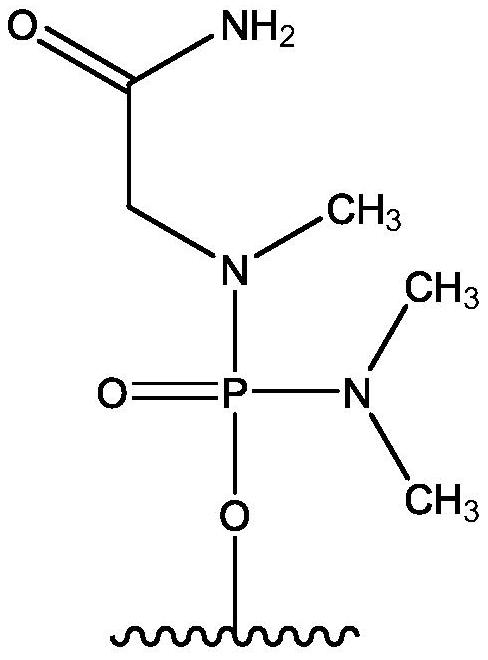
[0192] Quasimosen (also known as SRP-4045) is a PMO of the chemical structure described herein and is supplied by...
Embodiment 2
[0232] The primary objectives of the study are to assess the safety and tolerability of simoxan and to evaluate the pharmacokinetics (PK ).
[0233] method
[0234] A multicenter, randomized, double-blind, placebo-controlled, dose-titration, phase 1 / 2 study enrolled patients with advanced DMD and a confirmed mutation suitable for exon 45 skipping.
[0235] During double-blind dose titration, patients were randomized (2:1) to receive quasimosen or placebo for approximately 12 weeks. Patients randomized to receive quasimosen received 4 escalating dose levels (4, 10, 20, and 30 mg / kg), administered once weekly by intravenous (IV) infusion, for ≥2 weeks at each dose level. Following the double-blind dose-titration period, the safety and efficacy of once-weekly 30 mg / kg quasimoxan was assessed in an open-label extension period of up to an additional 132 weeks.
[0236] Patient: Eligibility
[0237] Eligible patients were males aged 7-21 years with a clinical diagnosis of DMD, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com