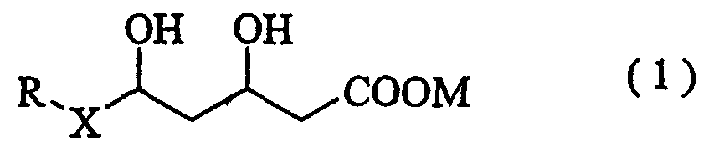Preventives and remedies for complications of diabetes
A therapeutic agent and technology for diabetes, applied in the field of prevention and treatment of diabetic complications, can solve the problem of unknown expression of osteopontin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Inhibition of Kidney and Vascular Osteopontin mRNA Expression in Diabetic Rats Induced by Streptozotocin (STZ)
[0026] The effect of pitavastatin calcium on the expression of osteopontin mRNA in the kidneys and blood vessels of streptozotocin (STZ)-induced diabetic rats was determined by the following method.
[0027] That is, for Wistar rats (body weight: about 300 g), 35 mg of STZ dissolved in physiological saline at a concentration of 50 mg / mL was injected per 1 kg of body weight through the tail vein, and about 1 hour later, 1 mL (3 mg / mL) per 1 kg of body weight was injected using a gastric probe. / kg) A 0.5% carboxymethylcellulose solution (3 mg / kg) of the drug to be tested (pitavastatin calcium) was forcibly orally administered. Then, the same amount of medicine was compulsorily orally administered once a day. In the control group, only the same amount of 0.5% carboxymethylcellulose solution was forcibly orally administered. On the second day of the...
Embodiment 2
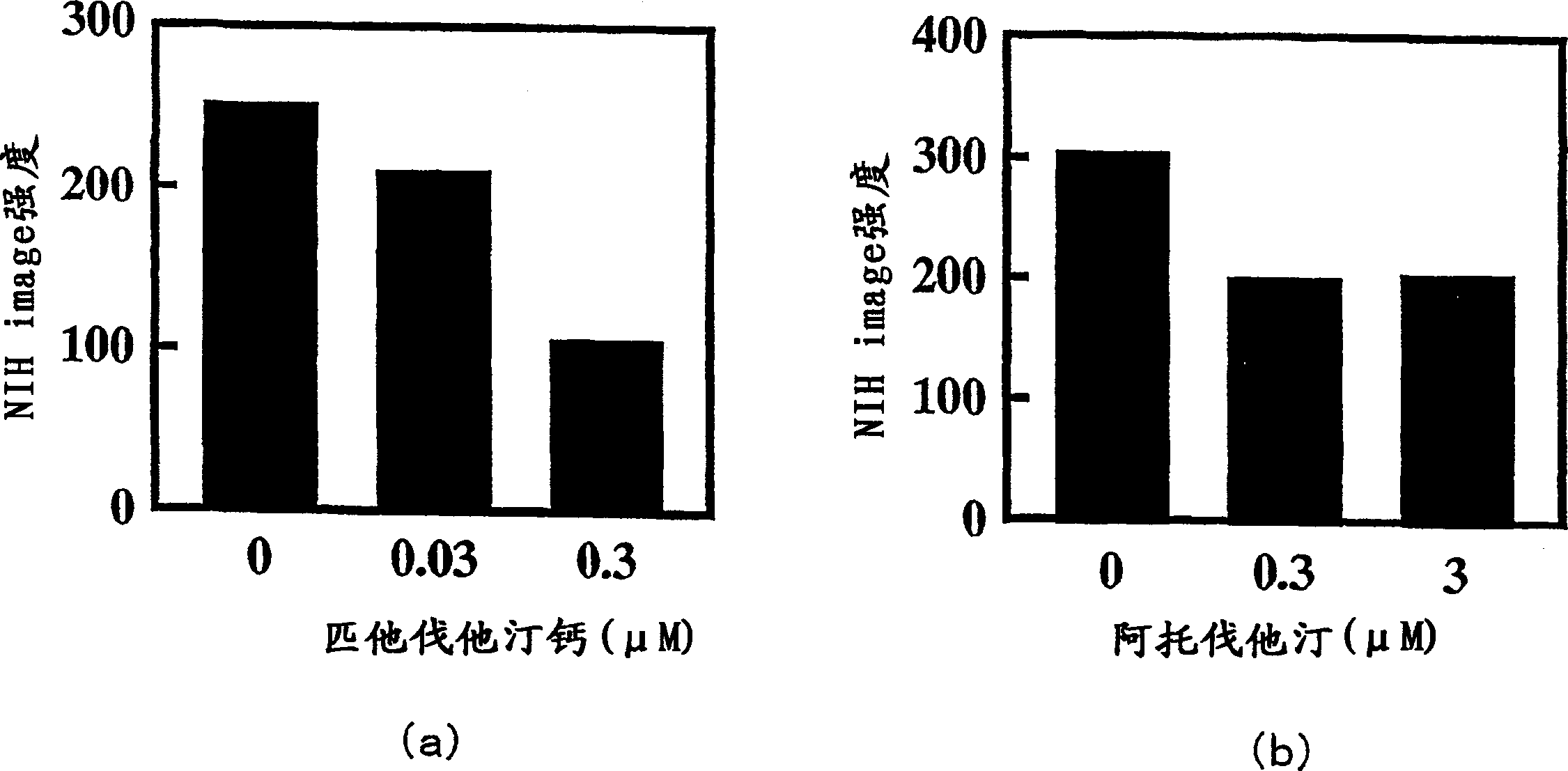
[0039] Example 2: Inhibition of Osteopontin Protein Secretion in Rat Aortic Smooth Muscle Cells
[0040] The effect of pitavastatin calcium and atorvastatin on the protein secretion of osteopontin in the culture supernatant of rat aortic smooth muscle cells cultured at normal glucose concentration was measured by the following method.
[0041] First, rat aortic smooth muscle cells (subcultured for 5 to 10 passages) were planted on a 6-well culture dish, and Eagle's Dulbecco's modified with low glucose (1000 mg / L) containing 10% fetal bovine serum (FBS: manufactured by BioWhittaker) was used. The culture medium (DMEM) was cultured confluently under the atmosphere of 5% CO2 and 37°C. Then, it was replaced with the same medium to which the test drugs (pitavastatin calcium and atorvastatin) were added, and cultured for 48 hours. Each well was replaced with 1.5 mL of the same medium not containing FBS, cultured for an additional 48 hours, and the supernatant was recovered. Add an...
Embodiment 3
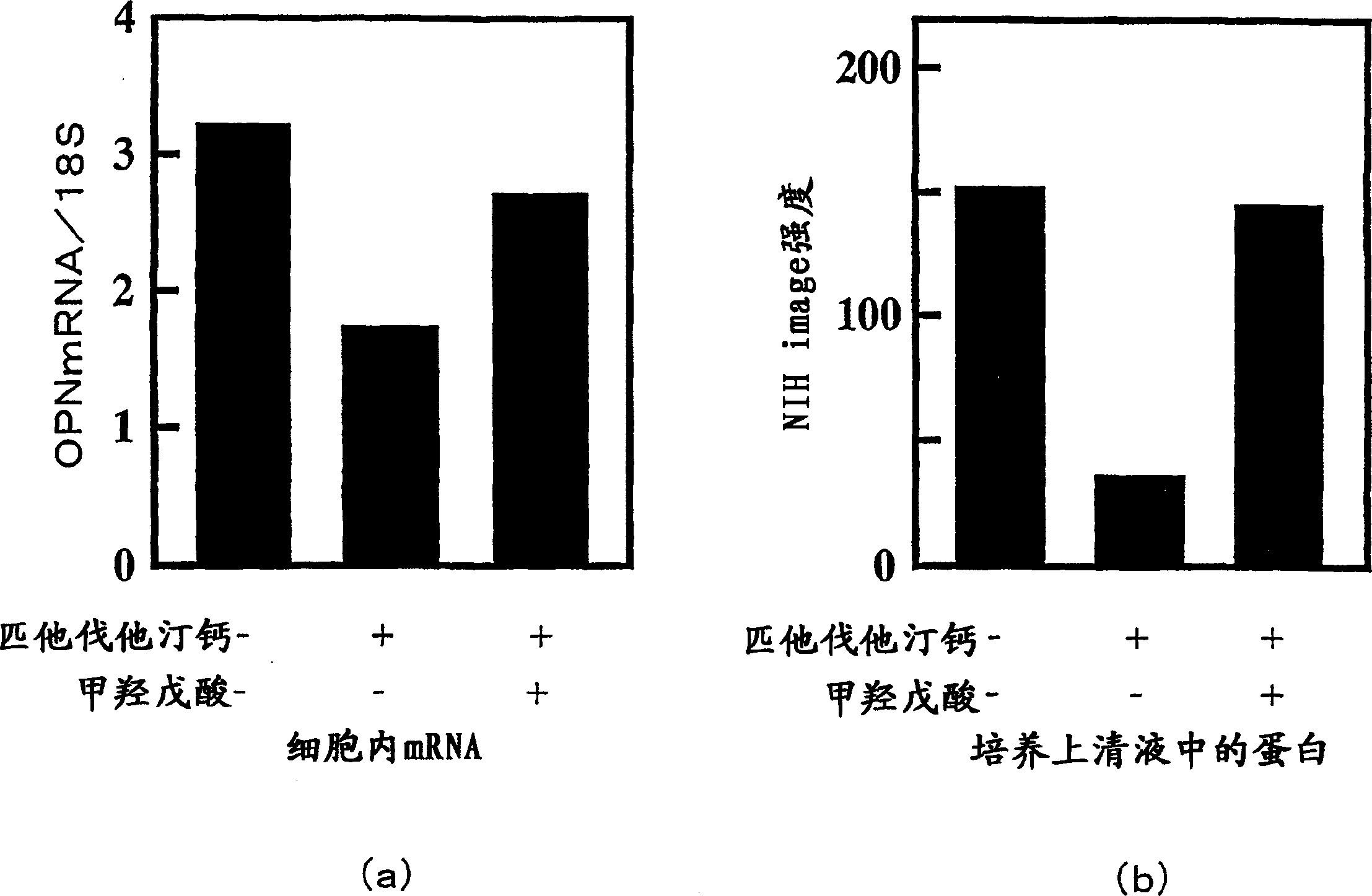
[0047] Example 3: Effects of Mevalonate on Osteopontin mRNA Expression and Osteopontin Protein Secretion Inhibition of Rat Aortic Smooth Muscle Cells
[0048] The effect of mevalonate on pitavastatin calcium inhibiting the expression of osteopontin mRNA in rat aortic smooth muscle cells cultured at normal glucose concentrations and the protein secretion of osteopontin protein in the culture supernatant, using the following Method determination.
[0049] Rat aortic smooth muscle cells (subcultured for 5 to 10 generations) were planted on 6-well culture dishes, and treated with low glucose (1000 mg / L) DMEM containing 10% FBS in 5% CO 2 , Confluent culture at 37°C. Then, it was replaced with the same medium supplemented with pitavastatin calcium (8 μM) and / or mevalonate (100 μM), and cultured for 48 hours. Each well was replaced with 1.5 mL of the same medium not containing FBS, and cultured for another 48 hours.
[0050] After culturing, the cells attached to the culture dish...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com