Glycosaminoglycans derived from K5 polysaccharide having high antithrombin activity and process for their preparation
一种糖胺聚糖、多糖的技术,应用在糖胺聚糖及其制备领域,能够解决产物没有、损失N-硫酸根等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention relates to a preparation method of K5 glycosaminoglycan, which comprises the following steps: (i) N-deacetylation / N-sulfation of polysaccharide K5, (ii) glucuronic acid moiety to corresponding iduraldehyde Partial C5 epimerization of the carboxyl group of the acid moiety, (iii) oversulfation, (iv) selective O-desulfation, (v) optional 6-O-sulfation, (vi) N-sulfation, wherein step (iv) comprises treating the oversulfated product obtained at the end of step (iii) with a mixture of methanol / dimethylsulfoxide for 135-165 minutes.
[0029] The treatment time is preferably about 150 minutes.
[0030] The product of the invention obtained from steps (ii) to (vi) can be chemically depolymerized by the method described in WO 82 / 03627, preferably after step (vi).
[0031] According to a preferred embodiment, the treatment of the oversulfated product obtained at the end of step (iii) with a mixture of methanol / dimethylsulfoxide is carried out at about 60° C....
Embodiment 1
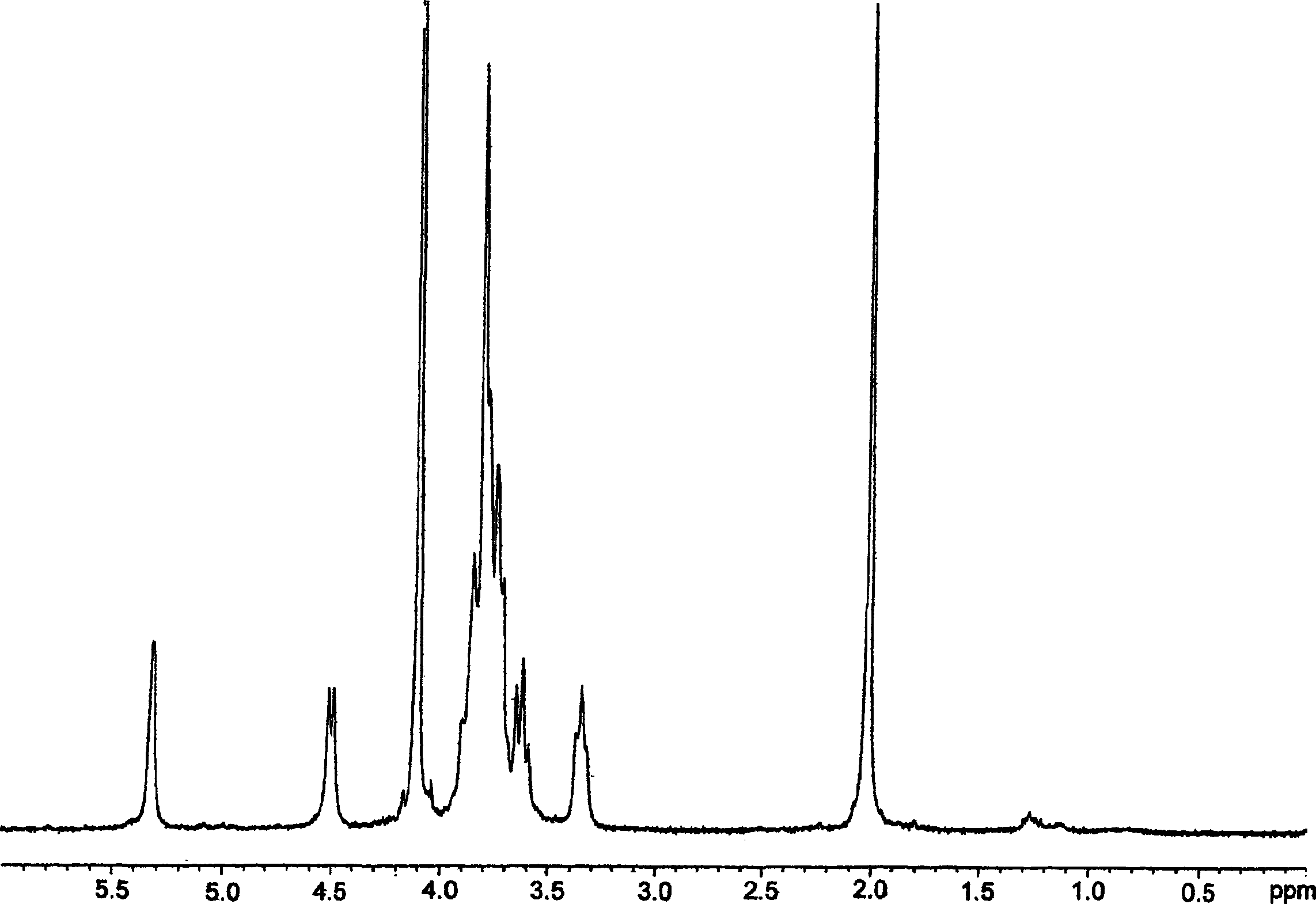
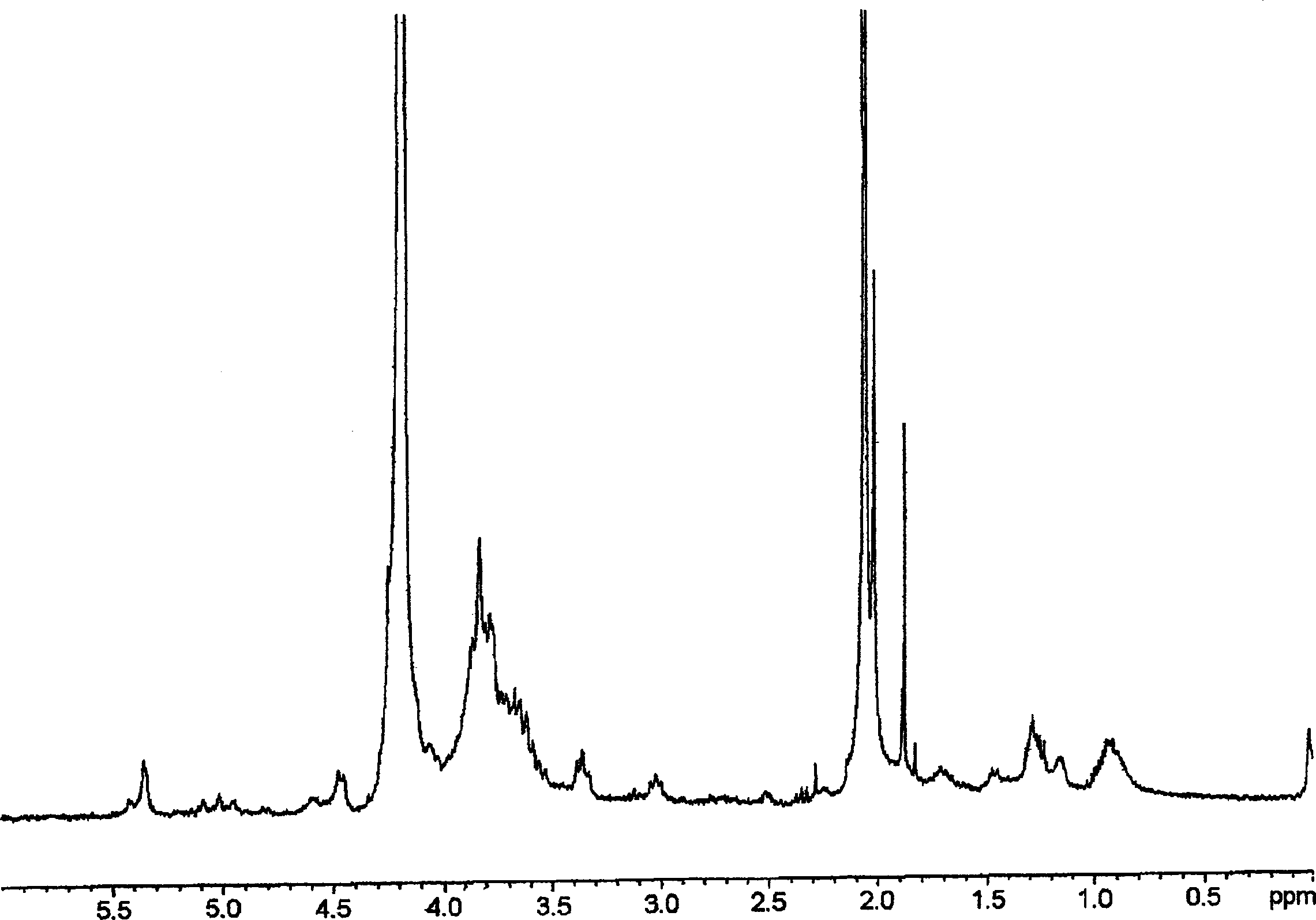
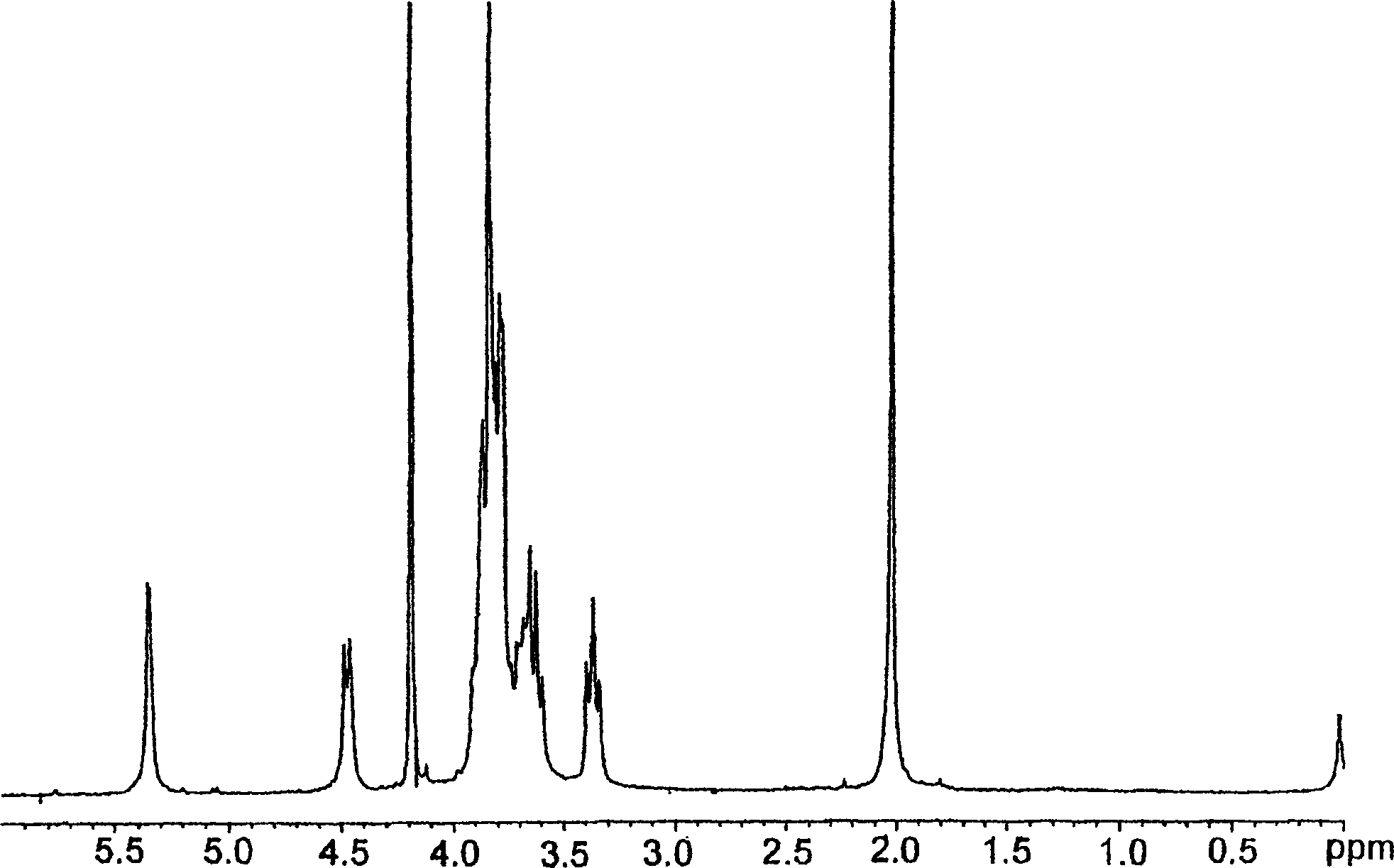
[0177] As described in Italian patent application MI99A001465 (WO 01 / 02597), 10 g of K5 polysaccharide was prepared by fermentation with a purity of 80% ( figure 2 ), these polysaccharides were dissolved in deionized water to prepare a 1% solution. Triton X-100 was added to bring the concentration to 5%, and the solution was stirred at 55°C for 2 hours. The temperature of the solution was raised to 75°C, maintained at this temperature until a homogeneous cloudy system was obtained, and the solution was rapidly cooled to room temperature. Two phases formed during cooling. The upper phase (organic phase) repeats the heat treatment twice. Finally, the aqueous phase containing K5 was concentrated to 1 / 10 under reduced pressure, and precipitated with acetone or ethanol. Discard the organic phase.
[0178] The resulting product was detected by proton NMR and compared with the spectrogram of the working standard ( figure 1 ) for comparison, it is determined to be K5 with a puri...
Embodiment 2
[0204] The C5-epimerized N,O-sulfated K5 obtained at the end of step (vi) of Example 1 was subjected to nitrous acid depolymerization under controlled conditions as described in WO 82 / 03627. More specifically, 5 g of a sample was dissolved in 250 ml of water, and cooled to 4°C using a constant temperature bath. Adjust the pH to 2 with 1N hydrochloric acid pre-cooled to 4°C, then add 10 ml of 1% sodium nitrite solution, if necessary, adjust the pH to 2 with 1N hydrochloric acid. The mixture was stirred slowly for 15 minutes, and the solution was neutralized with 1N sodium hydroxide precooled to 4°C, then 250 mg of sodium borohydride dissolved in 13 ml of deionized water was added, and stirred slowly for 4 hours. The pH of the mixture was adjusted to 5 with 1N hydrochloric acid, and the mixture was stirred for another 10 minutes to destroy excess sodium borohydride, and finally neutralized with 1N sodium hydroxide. The product was recovered by precipitation with 3 volumes of et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com