Nanometer grainy and mucosal immunity of antiatherosclerosis nucleic vaccine
An atherosclerotic nucleic acid, mucosal immunity technology, applied in the direction of antibody medical ingredients, medical preparations containing active ingredients, allergic diseases, etc., can solve problems such as hindering the development of such vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
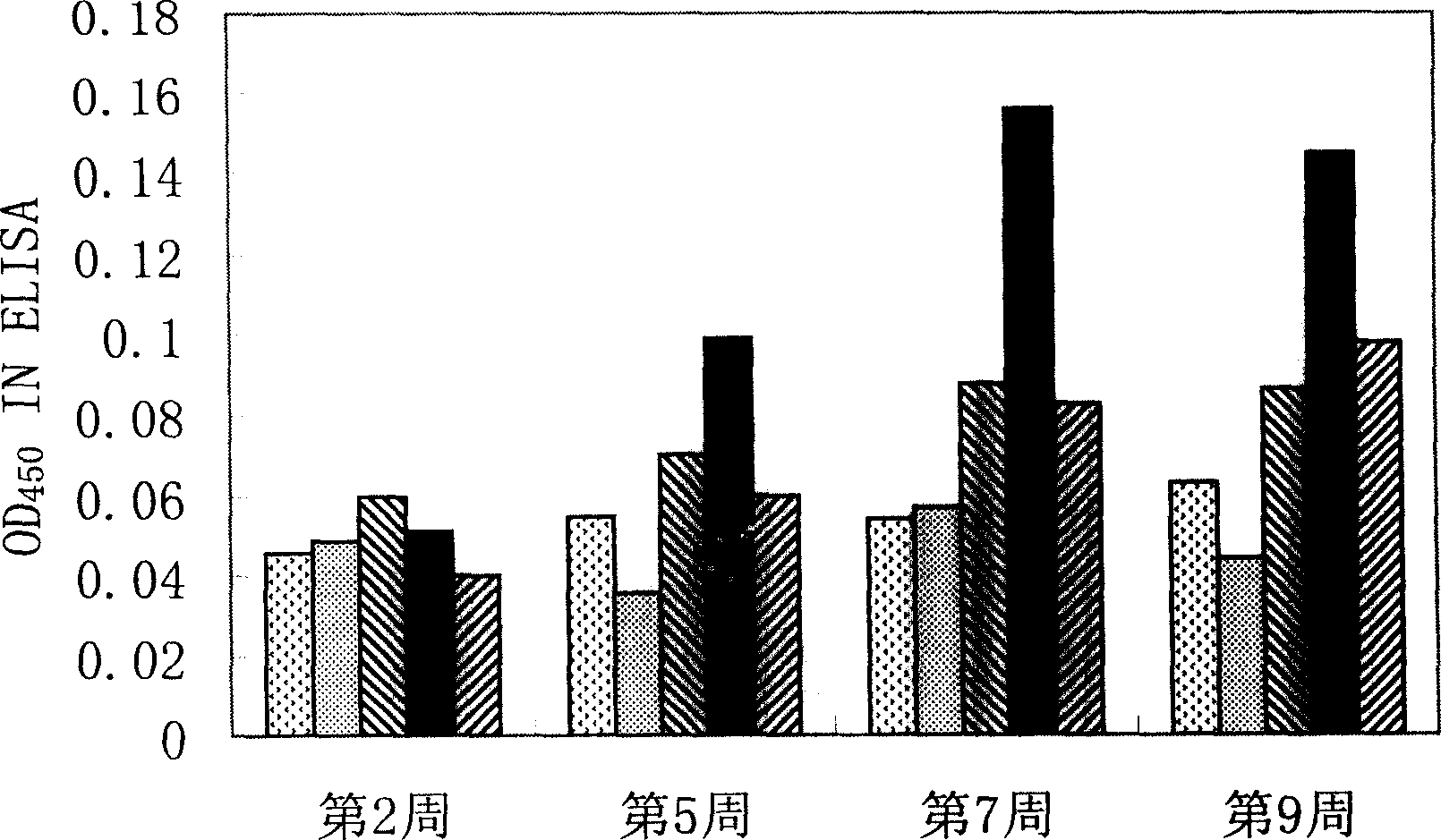
Image
Examples
Embodiment 1
[0019] An appropriate amount of chitosan was dissolved in 5 mM acetic acid-sodium acetate buffer solution (pH5.5) to prepare a solution with a concentration of 2 mg / ml. An appropriate amount of anti-atherosclerosis nucleic acid vaccine was dissolved in 15mM sodium sulfate solution to prepare a solution with a concentration of 500 μg / ml. Heat the above two solutions in a water bath at 55°C for 5 minutes, spin up 1 volume of the nucleic acid vaccine solution with a vortex shaker, quickly pour 0.5 volume of chitosan solution into the nucleic acid vaccine solution, and vortex for 20 seconds. Stand still at room temperature for more than 1 hour to obtain the colloidal solution of anti-atherosclerosis nucleic acid vaccine / chitosan nanoparticles.
Embodiment 2
[0021] An appropriate amount of chitosan was dissolved in 5 mM acetic acid-sodium acetate buffer solution (pH 5.5) to prepare a solution with a concentration of 300 μg / ml. An appropriate amount of anti-atherosclerosis nucleic acid vaccine was dissolved in 15mM sodium sulfate solution to prepare a solution with a concentration of 100 μg / ml. The above two solutions were heated in a water bath at 55° C. for 5 minutes, mixed rapidly with equal volumes, vortexed for 30 seconds, and left at room temperature for 1 hour to obtain a colloidal solution of anti-atherosclerotic nucleic acid vaccine / chitosan nanoparticles.
Embodiment 3
[0023] An appropriate amount of chitosan was dissolved in 5 mM acetic acid-sodium acetate buffer solution (pH5.5) to prepare a solution with a concentration of 2 mg / ml. An appropriate amount of anti-atherosclerosis nucleic acid vaccine was dissolved in 6.25mM sodium sulfate solution to prepare a solution with a concentration of 800 μg / ml. The above two solutions were heated in a water bath at 55° C. for 5 minutes, mixed rapidly with equal volumes, vortexed for 30 seconds, and left at room temperature for 1 hour to obtain a colloidal solution of anti-atherosclerotic nucleic acid vaccine / chitosan nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com